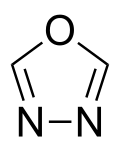
1,3,4-Oxadiazole is a nitrogen and oxygen containing heterocycle, and one of the four isomers of oxadiazole.[1][2]
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,3,4-Oxadiazole | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H2N2O | |
| Molar mass | 70.051 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Derivatives
edit1,3,4-Oxadiazole itself is not commonly used in organic chemistry, but many of its derivatives are important. For example, raltegravir is an HIV drug which contains an 1,3,4-oxadiazole ring. Other pharmaceutical drugs containing the 1,3,4-oxadiazole ring include fenadiazole, zibotentan, and tiodazosin.
1,3,4-Oxadiazole derivatives can be synthesized in a variety of ways.[3] One pathway is from oxidation of tetrazoles in the presence of aldehydes.[4] Similarly, the reaction of tetrazoles with acyl chlorides provides oxadiazoles.[5] Both methods involve the release of N2.
See also
edit- Furazan (1,2,5-oxadiazole)
References
edit- ^ PubChem. "1,3,4-Oxadiazole". pubchem.ncbi.nlm.nih.gov. National Center for Biotechnology Information, U.S. National Library of Medicine. Retrieved 2019-07-22.
- ^ Bala, Suman; Kamboj, Sunil; Kajal, Anu; Saini, Vipin; Prasad, Deo Nanadan (2014). "1,3,4-Oxadiazole Derivatives: Synthesis, Characterization, Antimicrobial Potential, and Computational Studies". BioMed Research International. 2014: 172791. doi:10.1155/2014/172791. PMC 4131560. PMID 25147788.
- ^ "1,3,4-Oxadiazole synthesis". www.organic-chemistry.org. Retrieved 11 November 2018.
- ^ Wang, Liang; Cao, Jing; Chen, Qun; He, Mingyang (17 April 2015). "One-Pot Synthesis of 2,5-Diaryl 1,3,4-Oxadiazoles via Di-tert-butyl Peroxide Promoted Acylation of Aryl Tetrazoles with Aldehydes". The Journal of Organic Chemistry. 80 (9): 4743–4748. doi:10.1021/acs.joc.5b00207. PMID 25860162.
- ^ Wong, Michael Y.; Krotkus, Simonas; Copley, Graeme; Li, Wenbo; Murawski, Caroline; Hall, David; Hedley, Gordon J.; Jaricot, Marie; Cordes, David B.; Slawin, Alexandra M. Z.; Olivier, Yoann; Beljonne, David; Muccioli, Luca; Moral, Monica; Sancho-Garcia, Juan-Carlos; Gather, Malte C.; Samuel, Ifor D. W.; Zysman-Colman, Eli (7 September 2018). "Deep-Blue Oxadiazole-Containing Thermally Activated Delayed Fluorescence Emitters for Organic Light-Emitting Diodes". ACS Applied Materials & Interfaces. 10 (39): 33360–33372. doi:10.1021/acsami.8b11136. hdl:10023/18433. PMID 30192504.