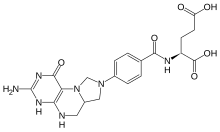
5,10-Methylenetetrahydrofolate (N5,N10-Methylenetetrahydrofolate; 5,10-CH2-THF) is cofactor in several biochemical reactions. It exists in nature as the diastereoisomer [6R]-5,10-methylene-THF.
| |
| Names | |
|---|---|
| IUPAC name
N-[4-(3-amino-1-oxo-1,4,5,6,6a,7-hexahydroimidazo[1,5-f]pteridin-8(9H)-yl)benzoyl]-L-glutamic acid
| |
| Other names
5,10-CH2-THF,
MTHF | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | 5,10-methylenetetrahydrofolate |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H23N7O6 | |
| Molar mass | 457.44 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
As an intermediate in one-carbon metabolism, 5,10-CH2-THF converts to 5-methyltetrahydrofolate, 5-formyltetrahydrofolate, and methenyltetrahydrofolate. It is substrate for the enzyme methylenetetrahydrofolate reductase (MTHFR)[1][2] It is mainly produced by the reaction of tetrahydrofolate with serine, catalyzed by the enzyme serine hydroxymethyltransferase.
Selected functions
editFormaldehyde equivalent
editMethylenetetrahydrofolate is a source of the equivalent of formaldehyde or CH22+ in biosyntheses.
Methylenetetrahydrofolate is also an intermediate in the detoxification of formaldehyde.[3]
Pyrimidine biosynthesis
editIt is the one-carbon donor for thymidylate synthase, for methylation of 2-deoxy-uridine-5-monophosphate (dUMP) to 2-deoxy-thymidine-5-monophosphate (dTMP). The coenzyme is necessary for the biosynthesis of thymidine and is the C1-donor in the reactions catalyzed by TS and thymidylate synthase (FAD).
Biomodulator
edit[6R]-5,10-methylene-THF is a biomodulator that has proven to enhance the desired cytotoxic antitumor effect of Fluorouracil (5-FU) and can bypass the metabolic pathway required by other folates (such as leucovorin) to achieve necessary activation.[4] The active metabolite is being evaluated in clinical trials for patients with colorectal cancer in combination with 5-FU.
See also
editReferences
edit- ^ "Entrez Gene: MTHFR methylenetetrahydrofolate reductase (NAD(P)H)".
- ^ Födinger M, Hörl WH, Sunder-Plassmann G (2000). "Molecular biology of 5,10-methylenetetrahydrofolate reductase". J Nephrol. 13 (1): 20–33. PMID 10720211.
- ^ Marx, C. J.; Chistoserdova, L.; Lidstrom, M. E. (2003). "Formaldehyde-detoxifying role of the tetrahydromethanopterin-linked pathway in Methylobacterium extorquens AM1". J. Bacteriol. 185 (24): 7160–8. doi:10.1128/jb.185.23.7160-7168.2003. PMC 296243. PMID 14645276.
- ^ Danenberg, Peter V.; Gustavsson, Bengt; Johnston, Patrick; Lindberg, Per; Moser, Rudolf; Odin, Elisabeth; Peters, Godefridus J.; Petrelli, Nicholas (2016-10-01). "Folates as adjuvants to anticancer agents: Chemical rationale and mechanism of action". Critical Reviews in Oncology/Hematology. 106: 118–131. doi:10.1016/j.critrevonc.2016.08.001. ISSN 1879-0461. PMID 27637357.