Tioguanine, also known as thioguanine or 6-thioguanine (6-TG) or tabloid is a medication used to treat acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), and chronic myeloid leukemia (CML).[2] Long-term use is not recommended.[2] It is given by mouth.[2]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Lanvis, Tabloid, others |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a682099 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 30% (range 14% to 46%) |
| Metabolism | Intracellular |
| Elimination half-life | 80 minutes (range 25–240 minutes) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.299 |
| Chemical and physical data | |
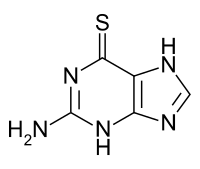
| Formula | C5H5N5S |
| Molar mass | 167.19 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Common side effects include bone marrow suppression, liver problems and inflammation of the mouth.[2][3] It is recommended that liver enzymes be checked weekly when on the medication.[2] People with a genetic deficiency in thiopurine S-methyltransferase are at higher risk of side effects.[3] Avoiding pregnancy when on the medication is recommended.[2] Tioguanine is in the antimetabolite family of medications.[3] It is a purine analogue of guanine and works by disrupting DNA and RNA.[4]
Tioguanine was developed between 1949 and 1951.[5][6] It is on the World Health Organization's List of Essential Medicines.[7]
Medical uses
edit- Acute leukemias in both adults and children
- Chronic myelogenous leukemia
- Inflammatory bowel disease, especially ulcerative colitis
- Psoriasis[8]
- Colorectal cancer in mice resistant to immunotherapy[9]
Side effects
edit- Leukopenia and neutropenia
- Thrombocytopenia
- Anemia
- Anorexia
- Nausea and vomiting
- Hepatotoxicity: this manifests as:
Hepatic veno-occlusive disease
editThe major concern that has inhibited the use of thioguanine has been veno-occlusive disease (VOD) and its histological precursor nodular regenerative hyperplasia (NRH). The incidence of NRH with thioguanine was reported as between 33 and 76%.[10] The risk of ensuing VOD is serious and frequently irreversible so this side effect has been a major concern. However, recent evidence using an animal model for thioguanine-induced NRH/VOD has shown that, contrary to previous assumptions, NRH/VOD is dose dependent and the mechanism for this has been demonstrated.[11] This has been confirmed in human trials, where thioguanine has proven to be safe but efficacious for coeliac disease when used at doses below those commonly prescribed.[12] This has led to a revival of interest in thioguanine because of its higher efficacy and faster action compared to other thiopurines and immunosuppressants such as mycophenylate.[13]
Contraindications
editInteractions
editCancers that do not respond to treatment with mercaptopurine do not respond to thioguanine. On the other hand, some cases of IBD that are resistant to mercaptopurine (or its pro-drug azathioprine) may be responsive to thioguanine.
Pharmacogenetics
editThe enzyme thiopurine S-methyltransferase (TPMT) is responsible for the direct inactivation of thioguanine to its methylthioguanine base – this methylation prevents thioguanine from further conversion into active, cytotoxic thioguanine nucleotide (TGN) metabolites.[15][16][17] Certain genetic variations within the TPMT gene can lead to decreased or absent TPMT enzyme activity, and individuals who are homozygous or heterozygous for these types of genetic variations may have increased levels of TGN metabolites and an increased risk of severe bone marrow suppression (myelosuppression) when receiving thioguanine.[15] In many ethnicities, TPMT polymorphisms that result in decreased or absent TPMT activity occur with a frequency of approximately 5%, meaning that about 0.25% of patients are homozygous for these variants.[15][18] However, an assay of TPMT activity in red blood cells or a TPMT genetic test can identify patients with reduced TPMT activity, allowing for the adjustment of thiopurine dose or avoidance of the drug entirely.[15][19] The FDA-approved drug label for thioguanine notes that patients who are TPMT-deficient may be prone to developing myelosuppression and that laboratories offer testing for TPMT deficiency.[20] Indeed, testing for TPMT activity is currently one of the few examples of pharmacogenetics being translated into routine clinical care.[21]
Metabolism and pharmacokinetics
editA single oral dose of thioguanine has incomplete metabolism, absorption and high interindividual variability. The bioavailability of thioguanine has an average of 30% (range 14-46%). The maximum concentration in plasma after a single oral dose is attained after 8 hours.
Thioguanine, like other thiopurines, is cytotoxic to white cells; as a result it is immunosuppressive at lower doses and anti-leukemic/anti-neoplastic at higher doses. Thioguanine is incorporated into human bone marrow cells, but like other thiopurines, it is not known to cross the blood-brain barrier. Thioguanine cannot be demonstrated in cerebrospinal fluid, similar to the closely related compound 6-mercaptopurine which also cannot penetrate to the brain.
The plasma half-life of thioguanine is short, due to the rapid uptake into liver and blood cells and conversion to 6-TGN. The median plasma half-life of 80-minutes with a range of 25–240 minutes. Thioguanine is excreted primarily through the kidneys in urine, but mainly as a metabolite, 2-amino-6-methylthiopurine. However, the intra-cellular thio-nucleotide metabolites of thioguanine (6-TGN) have longer half-lives and can therefore be measured after thioguanine is eliminated from the plasma.
Thioguanine is catabolized (broken down) via two pathways.[22] One route is through the deamination by the enzyme guanine deaminase to 6-thioxanthine, which has minimal anti-neoplastic activity, then by oxidation by xanthine oxidase of the thioxanthine to thiouric acid. This metabolic pathway is not dependent on the efficacy of xanthine oxidase, so that the inhibitor of xanthine oxidase, the drug allopurinol, does not block the breakdown of thioguanine, in contrast to its inhibition of the breakdown of the related thiopurine 6-mercaptopurine. The second pathway is the methylation of thioguanine to 2-amino-6-methylthiopurine, which is minimally effective as an anti-neoplastic and significantly less toxic than thioguanine. This pathway also is independent of the enzyme activity of xanthine oxidase.
Mechanism of action
edit6-Thioguanine is a thio analogue of the naturally occurring purine base guanine. 6-thioguanine utilises the enzyme hypoxanthine-guanine phosphoribosyltransferase (HGPRTase) to be converted to 6-thioguanosine monophosphate (TGMP). High concentrations of TGMP may accumulate intracellularly and hamper the synthesis of guanine nucleotides via the enzyme Inosine monophosphate dehydrogenase (IMP dehydrogenase), leading to DNA mutations.[23]
TGMP is converted by phosphorylation to thioguanosine diphosphate (TGDP) and thioguanosine triphosphate (TGTP). Simultaneously deoxyribosyl analogs are formed, via the enzyme ribonucleotide reductase. The TGMP, TGDP and TGTP are collectively named 6-thioguanine nucleotides (6-TGN). 6-TGN are cytotoxic to cells by: (1) incorporation into DNA during the synthesis phase (S-phase) of the cell; and (2) through inhibition of the GTP-binding protein (G protein) Rac1, which regulates the Rac/Vav pathway.[24]
Chemistry
editIt is a pale yellow, odorless, crystalline powder.
Names
editTioguanine (INN, BAN, AAN), or thioguanine (USAN).
Thioguanine is administered by mouth (as a tablet – 'Lanvis').
References
edit- ^ "Product monograph brand safety updates". Health Canada. February 2024. Retrieved 24 March 2024.
- ^ a b c d e f British National Formulary: BNF 69 (69th ed.). British Medical Association. 2015. pp. 588, 592. ISBN 978-0-85711-156-2.
- ^ a b c "Tioguanine 40 mg Tablets – Summary of Product Characteristics (SPC) – (eMC)". www.medicines.org.uk. Archived from the original on 21 December 2016. Retrieved 21 December 2016.
- ^ Baca QJ, Coen DM, Golan DE (2011). "Principles of antimicrobial and antineoplastic therapy". In Golan DE, Tashjian AH, Armstrong EJ (eds.). Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Lippincott Williams & Wilkins. p. 686. ISBN 978-1-60831-270-2. Archived from the original on 2016-12-21.
- ^ Dubler E (1996). "Metal Complexes of Sulfur-Containing Purine Derivatives". In Sigel A, Sigel H (eds.). Metal Ions in Biological Systems. Vol. 32: Interactions of Metal Ions with Nucleotides: Nucleic Acids, and Their Constituents. CRC Press. p. 302. ISBN 978-0-8247-9549-8. Archived from the original on 2016-12-21.
- ^ Landau R, Achilladelis B, Scriabine A (1999). "Ch. 6. Clinical champions as critical determinants of drug development.". Pharmaceutical Innovation: Revolutionizing Human Health. Chemical Heritage Foundation. p. 342. ISBN 978-0-941901-21-5. Archived from the original on 2016-12-21.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Mason C, Krueger GG (January 2001). "Thioguanine for refractory psoriasis: a 4-year experience". Journal of the American Academy of Dermatology. 44 (1): 67–72. doi:10.1067/mjd.2001.109296. PMID 11148479.
- ^ Amodio V, Lamba S, Chilà R, Cattaneo CM, Mussolin B, Corti G, et al. (January 2023). "Genetic and pharmacological modulation of DNA mismatch repair heterogeneous tumors promotes immune surveillance". Cancer Cell. 41 (1): 196–209.e5. doi:10.1016/j.ccell.2022.12.003. PMC 9833846. PMID 36584674.
- ^ Dubinsky MC, Vasiliauskas EA, Singh H, Abreu MT, Papadakis KA, Tran T, et al. (August 2003). "6-thioguanine can cause serious liver injury in inflammatory bowel disease patients". Gastroenterology. 125 (2): 298–303. doi:10.1016/S0016-5085(03)00938-7. PMID 12891528.
- ^ Oancea I, Png CW, Das I, Lourie R, Winkler IG, Eri R, et al. (April 2013). "A novel mouse model of veno-occlusive disease provides strategies to prevent thioguanine-induced hepatic toxicity". Gut. 62 (4): 594–605. doi:10.1136/gutjnl-2012-302274. PMID 22773547. S2CID 29585979.
- ^ Tack GJ, van Asseldonk DP, van Wanrooij RL, van Bodegraven AA, Mulder CJ (August 2012). "Tioguanine in the treatment of refractory coeliac disease--a single centre experience". Alimentary Pharmacology & Therapeutics. 36 (3): 274–281. doi:10.1111/j.1365-2036.2012.05154.x. PMID 22646133. S2CID 24811114.
- ^ Van Asseldonk DP, Oancea I, Jharap B, et al. (March 2012). "Is thioguanine-associated sinusoidal obstruction syndrome avoidable? Lessons learned from 6-thioguanine treatment of inflammatory bowel disease and a mouse model" (PDF). Journal of the Brazilian Medical Association. 58 (Suppl.1): S8–13.
- ^ Gardiner SJ, Gearry RB, Roberts RL, Zhang M, Barclay ML, Begg EJ (October 2006). "Exposure to thiopurine drugs through breast milk is low based on metabolite concentrations in mother-infant pairs". British Journal of Clinical Pharmacology. 62 (4): 453–456. doi:10.1111/j.1365-2125.2006.02639.x. PMC 1885151. PMID 16995866.
- ^ a b c d Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW, et al. (March 2011). "Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing". Clinical Pharmacology and Therapeutics. 89 (3): 387–391. doi:10.1038/clpt.2010.320. PMC 3098761. PMID 21270794.
- ^ Zaza G, Cheok M, Krynetskaia N, Thorn C, Stocco G, Hebert JM, et al. (September 2010). "Thiopurine pathway". Pharmacogenetics and Genomics. 20 (9): 573–574. doi:10.1097/FPC.0b013e328334338f. PMC 3098750. PMID 19952870.
- ^ Fujita K, Sasaki Y (August 2007). "Pharmacogenomics in drug-metabolizing enzymes catalyzing anticancer drugs for personalized cancer chemotherapy". Current Drug Metabolism. 8 (6): 554–562. doi:10.2174/138920007781368890. PMID 17691917. Archived from the original on 2013-01-12.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ Mutschler E, Schäfer-Korting M (2001). Arzneimittelwirkungen (in German) (8th ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. pp. 107, 936. ISBN 978-3-8047-1763-3.
- ^ Payne K, Newman W, Fargher E, Tricker K, Bruce IN, Ollier WE (May 2007). "TPMT testing in rheumatology: any better than routine monitoring?". Rheumatology. 46 (5): 727–729. doi:10.1093/rheumatology/kel427. PMID 17255139.
- ^ "TABLOID- thioguanine tablet". DailyMed. Retrieved 17 March 2015.
- ^ Wang L, Pelleymounter L, Weinshilboum R, Johnson JA, Hebert JM, Altman RB, Klein TE (June 2010). "Very important pharmacogene summary: thiopurine S-methyltransferase". Pharmacogenetics and Genomics. 20 (6): 401–405. doi:10.1097/FPC.0b013e3283352860. PMC 3086840. PMID 20154640.
- ^ Oncea I, Duley J (2008). "Chapter 38. Pharmacogenetics of Thiopurines.". In Brunton LL, Lazo JS, Parker K (eds.). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). McGraw-Hill's Access Medicine (on-line).
- ^ Evans WE (April 2004). "Pharmacogenetics of thiopurine S-methyltransferase and thiopurine therapy". Therapeutic Drug Monitoring. 26 (2): 186–191. doi:10.1097/00007691-200404000-00018. PMID 15228163. S2CID 34015182.
- ^ de Boer NK, van Bodegraven AA, Jharap B, de Graaf P, Mulder CJ (December 2007). "Drug Insight: pharmacology and toxicity of thiopurine therapy in patients with IBD". Nature Clinical Practice. Gastroenterology & Hepatology. 4 (12): 686–694. doi:10.1038/ncpgasthep1000. PMID 18043678. S2CID 23686284.
Further reading
edit- Dean L (2012). "Thioguanine Therapy and TPMT Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28520351. Bookshelf ID: NBK100663.