Dihydroergocryptine (DHEC), sold under the brand names Almirid and Cripar among others, is a dopamine agonist of the ergoline group that is used as an antiparkinson agent in the treatment of Parkinson's disease.[1] It is taken by mouth.[citation needed]
 | |
| Clinical data | |
|---|---|
| Trade names | Almirid, Cripar |
| Other names | Dihydroergocriptine; DHEC; 12'-Hydroxy-2'-(1-methylethyl)-5'α-(2-methylpropyl)-9,10α-dihydroergotaman-3',6',18-trione; (5'α,10α)-9,10-Dihydro-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-ergotaman-3',6',18-trione |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 12–16 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.042.706 |
| Chemical and physical data | |
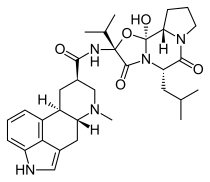
| Formula | C32H43N5O5 |
| Molar mass | 577.726 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Medical uses
editParkinson's disease
editDihydroergocryptine has been shown to be particularly effective as monotherapy in the early stages of Parkinson's disease. Initial monotherapy with a dopamine agonist (other examples include pergolide, pramipexole, and ropinirole) is associated with reduced risk for motor complications in Parkinson patients relative to levodopa.[2] DHEC, like other dopamine agonists, aims to mimic the endogenous neurotransmitter and exert an antiparkinsonian effect.[3] Recent evidence also supports that dopamine receptor agonists, instead of levodopa may slow or prevent the progression of Parkinson's disease.[4]
The relatively long half-life and lack of dietary influence of dihydroergocriptine is considered to contribute to the compound's effectiveness in Parkinson's disease, particularly since it allows for more continuous stimulation of brain dopaminergic receptors than short-acting drugs such as levodopa.[5] DHEC is also proven to be a safe and effective in improving symptoms in Parkinson's patients.[6]
Motor improvements in Parkinson's patients are usually observed in patients who take at least a mean daily dose of approximately 40 mg.[7] Patients on DHEC demonstrate a better score than if they were on levodopa on the Webster scale, a standardized rating scale of Parkinson's Disease symptoms such as gait parameters and dyskinesia.[5][8] Another clinical study has shown that DHEC had superior efficacy in reducing the clinical and motorcomplications associated with long-term levodopa use, as well as in reducing the incidence and severity of adverse effects.[1]
Activation of presynaptic dopamine autoreceptors by dihydroergocriptine leads to reduced dopamine receptor turnover and indirect antioxidant effects. In particular, further activation of intracellular kinase systems due to dopamine agonists are hypothesized to lead to antiapoptotic effects that also help in halting and slowing the disease progression.[2] This may also contribute to prevention of development of motor fluctuations, though more research is needed.[9]
Modern agonists like dihydroergocryptine typically cost two to three times more than levodopa therapy. More health economics assessments may be needed to determine whether the initial increased costs of the agonists are offset by less patients needing surgery in later stages of the disease.[10]
Other uses
editDihydroergocryptine can also be used in migraine prophylaxis,[11] as well as for the treatment of low blood pressure in elderly patients and peripheral vascular disorder.[12] More commonly, it is used in combination with two similar compounds, dihydroergocornine and dihydroergocristine. This mixture is called ergoloid or codergocrine.[13]
Side effects
editDihydroergocryptine has been suggested to produce fewer side-effects and have similar efficacy to a classical dopamine agonist due to its biochemical profile.[5] There is also no interference with levodopa metabolism.[10] Although DHEC may come with some acute side-effects described further below, DHEC has overall good tolerability with little to no withdrawal or changes in its scheduling.[7]
Acute side-effects usually accompany the beginning of treatment but tend to decrease as the patient develops increased tolerance to the drug.[14] In randomized, double-blinded trials, individuals on different dopamine agonists, including dihydroergocryptine, did not differ in discontinuation rate associated with adverse events.[15][16] However, there do seem to be a higher incidence of dopaminergic related side-effects such as hallucinations and gastrointestinal complaints tend to be more frequent.[6]
Pharmacology
editPharmacodynamics
editSeveral in vitro and in vivo studies have demonstrated that dihydroergocriptine is an effective anti-Parkinson drug, most likely exerting its effects as a potent agonist of D2 receptors. The Kd of DHEC is found to be around 5-8 nM at D2 receptors. Less certain is the contribution of its partial D1 receptor and D3 receptor agonist activity. DHEC has a lower affinity for D1 and D3 receptors (Kd is around 30 nM for both) than for D2 receptors.[3] It is widely believed that dopamine receptor agonists demonstrate their antiparkinsonian effects by stimulating D2 receptors primarily, but other dopamine receptors, such as D1 and D3 may be involved.[3]
Remarkably, DHEC is said to not significantly interact with serotonergic and adrenergic receptors.[5]
Pharmacokinetics
editDihydroergocriptine has two main pharmacokinetic advantages over levodopa.
The first pharmacokinetic advantage is its half-life of 12 to 16 hours. This relatively long half-life is considered to contribute to the compound's effectiveness in Parkinson's disease, particularly since it allows for more continuous stimulation of brain dopaminergic receptors than short-acting drugs such as levodopa. Though the exact reason is not known, continuous stimulation is considered to reduce risk for motor complications.[2]
The second pharmacokinetic advantage is the lack of dietary influence on drug absorption. This characteristic also allows for more sustained dopamine receptor stimulation.[5]
DHEC can be taken with a single oral dose and is rapidly absorbed. Peak plasma concentrations occur between 30 and 120 minutes after administration. The strong first-pass hepatic metabolism results in poor bioavailability. Less than 5% of the original dosage reaches the circulation.[5]
Chemistry
editDihydroergocryptine is a mixture of two very similar compounds, alpha- and beta-dihydroergocryptine (epicriptine) at a ratio of 2:1.[12] The beta differs from the alpha form only in the position of a single methyl group, which is a consequence of the biosynthesis of the parent compound ergocryptine, in which the proteinogenic amino acid leucine is replaced by isoleucine.[17]
Dihydroergocryptine is a hydrogenated ergot derivative that is also structurally very similar to bromocriptine, another drug that has anti-Parkinson effects. DHEC differs in that it is hydrogenated in C9–C10 and lacks bromine in C2. In fact, all ergot derivatives are uniquely or mainly D2-like receptor agonists.[5]
References
edit- ^ a b Battistin L, Bardin PG, Ferro-Milone F, Ravenna C, Toso V, Reboldi G (January 1999). "Alpha-dihydroergocryptine in Parkinson's disease: a multicentre randomized double blind parallel group study". Acta Neurologica Scandinavica. 99 (1): 36–42. doi:10.1111/j.1600-0404.1999.tb00655.x. PMID 9925236. S2CID 45192184.
- ^ a b c Antonini A, Tolosa E, Mizuno Y, Yamamoto M, Poewe WH (October 2009). "A reassessment of risks and benefits of dopamine agonists in Parkinson's disease". The Lancet. Neurology. 8 (10): 929–37. doi:10.1016/S1474-4422(09)70225-X. PMID 19709931. S2CID 33649811.
- ^ a b c Gerlach M, Double K, Arzberger T, Leblhuber F, Tatschner T, Riederer P (October 2003). "Dopamine receptor agonists in current clinical use: comparative dopamine receptor binding profiles defined in the human striatum". Journal of Neural Transmission. 110 (10): 1119–27. doi:10.1007/s00702-003-0027-5. PMID 14523624. S2CID 10073899.
- ^ Parkinson Study Group (April 2002). "Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression". JAMA. 287 (13): 1653–61. doi:10.1001/jama.287.13.1653. PMID 11926889.
- ^ a b c d e f g Albanese A, Colosimo C (May 2003). "Dihydroergocriptine in Parkinson's disease: clinical efficacy and comparison with other dopamine agonists". Acta Neurologica Scandinavica. 107 (5): 349–55. doi:10.1034/j.1600-0404.2003.02049.x. PMID 12713527. S2CID 18094044.
- ^ a b Bergamasco B, Frattola L, Muratorio A, Piccoli F, Mailland F, Parnetti L (June 2000). "Alpha-dihydroergocryptine in the treatment of de novo parkinsonian patients: results of a multicentre, randomized, double-blind, placebo-controlled study". Acta Neurologica Scandinavica. 101 (6): 372–80. doi:10.1034/j.1600-0404.2000.90295a.x. PMID 10877152. S2CID 9859381.
- ^ a b Martignoni E, Pacchetti C, Sibilla L, Bruggi P, Pedevilla M, Nappi G (February 1991). "Dihydroergocryptine in the treatment of Parkinson's disease: a six months' double-blind clinical trial". Clinical Neuropharmacology. 14 (1): 78–83. doi:10.1097/00002826-199102000-00006. PMID 1903079.
- ^ Ramaker C, Marinus J, Stiggelbout AM, Van Hilten BJ (September 2002). "Systematic evaluation of rating scales for impairment and disability in Parkinson's disease". Movement Disorders. 17 (5): 867–76. doi:10.1002/mds.10248. PMID 12360535. S2CID 2562332.
- ^ Olanow CW (February 1992). "A rationale for dopamine agonists as primary therapy for Parkinson's disease". The Canadian Journal of Neurological Sciences. 19 (1 Suppl): 108–12. doi:10.1017/S0317167100041469. PMID 1349262.
- ^ a b Clarke CE, Guttman M (November 2002). "Dopamine agonist monotherapy in Parkinson's disease". Lancet. 360 (9347): 1767–9. doi:10.1016/S0140-6736(02)11668-0. PMID 12480442. S2CID 25118777.
- ^ Micieli G, Cavallini A, Marcheselli S, Mailland F, Ambrosoli L, Nappi G (April 2001). "Alpha-dihydroergocryptine and predictive factors in migraine prophylaxis". International Journal of Clinical Pharmacology and Therapeutics. 39 (4): 144–51. doi:10.5414/cpp39144. PMID 11332869.
- ^ a b Haberfeld, H, ed. (2007). Austria-Codex (in German) (2007/2008 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 978-3-85200-183-8.
- ^ Drugs.com: Ergoloid Mesylates
- ^ Yamamoto M, Schapira AH (April 2008). "Dopamine agonists in Parkinson's disease". Expert Review of Neurotherapeutics. 8 (4): 671–7. doi:10.1586/14737175.8.4.671. PMID 18416667. S2CID 207194957.
- ^ Rascol O, Goetz C, Koller W, Poewe W, Sampaio C (May 2002). "Treatment interventions for Parkinson's disease: an evidence based assessment". Lancet. 359 (9317): 1589–98. doi:10.1016/S0140-6736(02)08520-3. PMID 12047983. S2CID 24426198.
- ^ Goetz CG, Poewe W, Rascol O, Sampaio C (May 2005). "Evidence-based medical review update: pharmacological and surgical treatments of Parkinson's disease: 2001 to 2004". Movement Disorders. 20 (5): 523–39. doi:10.1002/mds.20464. PMID 15818599. S2CID 16260982.
- ^ Steinhilber D, Schubert-Zsilavecz M, Roth HJ (2005). Medizinische Chemie (in German). Stuttgart: Deutscher Apotheker Verlag. p. 142. ISBN 978-3-7692-3483-1.