Ammonium bifluoride is an inorganic compound with the formula [NH4][HF2] or [NH4]F·HF. It is produced from ammonia and hydrogen fluoride. This colourless salt is a glass-etchant and an intermediate in a once-contemplated route to hydrofluoric acid.
| |||

| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Ammonium bifluoride
| |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.014.252 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| UN number | 1727 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| [NH4][HF2] | |||
| Molar mass | 57.044 g·mol−1 | ||
| Appearance | Colourless crystals | ||
| Density | 1.50 g cm−3 | ||
| Melting point | 126 °C (259 °F; 399 K) | ||
| Boiling point | 240 °C (464 °F; 513 K)(decomposes) | ||
| 63g/(100 ml) (20 °C) | |||
| Solubility in alcohol | slightly soluble | ||
Refractive index (nD)
|
1.390 | ||
| Structure | |||
| Cubic, related to the CsCl structure | |||
| [NH4]+ cation: tetrahedral [HF2]− anion: linear | |||
| Hazards | |||
| GHS labelling: | |||
  [1] [1]
| |||
| H301, H314[1] | |||
| P280, P301+P310, P305+P351+P338, P310[1] | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Other cations
|
potassium bifluoride | ||
Related compounds
|
ammonium fluoride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Structure
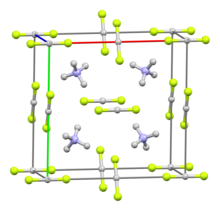
editAmmonium bifluoride, as its name indicates, contains an ammonium cation ([NH4]+), and a bifluoride (or hydrogen difluoride) anion ([HF2]−). The triatomic bifluoride anion features a strong three-center four-electron bond with a bond energy greater than 155 kJ/mol,[2] and an H-F length of 114 pm.[3]
In solid form ([NH4][HF2]), ammonium biflouride is similar to other fluoride salts.[4] Its crystal system is considered orthorhombic,[5] with each cation coordinated with four anions in a tetrahedron (and vice-versa). Hydrogen atoms in the ammonium ion form hydrogen bonds with the fluorine atoms, and in the resulting structure, N-H-F are roughly colinear.[6][7] As a result of these hydrogen bonds, this crystal structure varies from those of other bifluoride salts, such as potassium bifluoride and rubidium bifluoride.[5] Its crystals are unstable, and decompose rapidly when exposed to air.[6]
Production and applications
editAmmonium bifluoride is a component of some etchants. It attacks silica component of glass:
Potassium bifluoride is a related more commonly used etchant.
Ammonium bifluoride has been considered as an intermediate in the production of hydrofluoric acid from hexafluorosilicic acid. Thus, hexafluorosilicic acid is hydrolyzed to give ammonium fluoride, which thermally decomposes to give the bifluoride:
- H2[SiF6] + 6 NH3 + 2 H2O → SiO2 + 6 [NH4]F
- 2 [NH4]F → NH3 + [NH4][HF2]
The resulting ammonium bifluoride is converted to sodium bifluoride, which thermally decomposes to release HF.[8]
Ammonium bifluoride is also used as an additive in tin-nickel plating processes as the fluoride ion acts as a complexing agent with the tin, allowing for greater control over the resulting composition and finish.
Toxicity
editAmmonium bifluoride is toxic to consume and a skin corrosion agent. Upon exposure to skin, rinsing with water followed by a treatment of calcium gluconate is required.[1] In water, ammonium bifluoride exists in chemical equilibrium with hydrofluoric acid and heating releases hydrogen fluoride gas.[9] Consequently, there is an equivalent toxicological risk as is present with hydrofluoric acid, and the same safety precautions apply.[10][9]
Ammonium bifluoride is used in some automotive wheel cleaning products. Many injuries have resulted in users not being aware of the risks posed by the products.[11] Ammonium bifluoride based products are often considered a safer alternative to hydrofluoric acid, yet still pose clear risks to the handler.[10] Ammonium bifluoride, ammonium fluoride, and hydrofluoric acid have been described as "too dangerous for any use in a car wash environment" by Professional Car Washing and Detailing magazine,[12] advice that accords with a 2015 report from the U.S. Centers for Disease Control and Prevention.[13]
References
edit- ^ a b c d Sigma-Aldrich Co., Ammonium bifluoride. Retrieved on 2013-07-20.
- ^ Emsley, J. (1980) Very Strong Hydrogen Bonds, Chemical Society Reviews, 9, 91–124. doi:10.1039/CS9800900091
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ "Ammonium bifluoride". PubChem. Retrieved 17 September 2024.
- ^ a b Troyanov, S. I. (2005). "Crystal Structure Refinement of Bifluorides MHF2 (M = Na, NH4, Rb). Crystal Structures of Rb5F4(HF2) × 2H2O and RbF × H2O". Crystallography Reports. 50 (5): 773–778. doi:10.1134/1.2049394. Retrieved 17 September 2024.
- ^ a b McDonald, T. R. R. (1960). "The electron-density distribution in ammonium bifluoride". Acta Crystallogr. 13 (2): 113–124. Bibcode:1960AcCry..13..113M. doi:10.1107/S0365110X60000261.
- ^ "ICSD Entry: 14140". Cambridge Structural Database: Access Structures. Cambridge Crystallographic Data Centre. Retrieved 2022-06-25.
- ^ Aigueperse, Jean; Mollard, Paul; Devilliers, Didier; Chemla, Marius; Faron, Robert; Romano, René; Cuer, Jean Pierre (2000). "Fluorine Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_307. ISBN 3527306730.
- ^ a b National Industrial Chemicals Notification and Assessment Scheme (April 17, 2020). "Bifluorides: Human health tier II assessment" (PDF). Department of Health (Australia). Retrieved February 8, 2023.
- ^ a b Genuino, Homer C.; Opembe, Naftali N.; Njagi, Eric C.; McClain, Skye; Suib, Steven L. (2012). "A review of hydrofluoric acid and its use in the car wash industry". Journal of Industrial and Engineering Chemistry. 18 (5): 1529–1539. doi:10.1016/j.jiec.2012.03.001. ISSN 1226-086X.
- ^ Gormley, James (May 29, 2001). "The truth about ammonium bifluoride". Professional Car Washing and Detailing. Archived from the original on 2001-05-29.
- ^ Cook, Ryan (October 9, 2013). "The Five Factors of Clean: Chemistry, Part 1". Professional Car Washing and Detailing. Retrieved February 8, 2023.
- ^ Woodie, Maria (December 1, 2015). "OSHA gives employees the right to know". Professional Car Washing and Detailing. Retrieved February 8, 2023.


