A BRCA mutation is a mutation in either of the BRCA1 and BRCA2 genes, which are tumour suppressor genes. Hundreds of different types of mutations in these genes have been identified, some of which have been determined to be harmful, while others have no proven impact. Harmful mutations in these genes may produce a hereditary breast–ovarian cancer syndrome in affected persons. Only 5–10% of breast cancer cases in women are attributed to BRCA1 and BRCA2 mutations (with BRCA1 mutations being slightly more common than BRCA2 mutations), but the impact on women with the gene mutation is more profound.[2] Women with harmful mutations in either BRCA1 or BRCA2 have a risk of breast cancer that is about five times the normal risk, and a risk of ovarian cancer that is about ten to thirty times normal.[3] The risk of breast and ovarian cancer is higher for women with a high-risk BRCA1 mutation than with a BRCA2 mutation. Having a high-risk mutation does not guarantee that the woman will develop any type of cancer, or imply that any cancer that appears was actually caused by the mutation, rather than some other factor.
| BRCA mutation | |
|---|---|
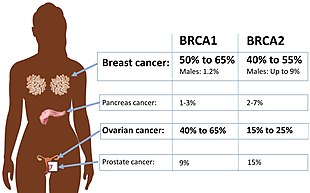 | |
| Absolute risk of cancers in BRCA1 or BRCA2 mutation.[1] | |
| Specialty | Medical genetics |
High-risk mutations, which disable an important error-free DNA repair process (homology directed repair), significantly increase the person's risk of developing breast cancer, ovarian cancer and certain other cancers. Why BRCA1 and BRCA2 mutations lead preferentially to cancers of the breast and ovary is not known, but lack of BRCA1 function seems to lead to non-functional X-chromosome inactivation. Not all mutations are high-risk; some appear to be harmless variations. The cancer risk associated with any given mutation varies significantly and depends on the exact type and location of the mutation and possibly other individual factors.
Mutations can be inherited from either parent and may be passed on to both sons and daughters. Each child of a genetic carrier, regardless of sex, has a 50% chance of inheriting the mutated gene from the parent who carries the mutation. As a result, half of the people with BRCA gene mutations are male, who would then pass the mutation on to 50% of their offspring, male or female. The risk of BRCA-related breast cancers for men with the mutation is higher than for other men, but still low.[4] However, BRCA mutations can increase the risk of other cancers, such as colon cancer, pancreatic cancer, and prostate cancer.
Methods to diagnose the likelihood of a patient with mutations in BRCA1 and BRCA2 getting cancer were covered by patents owned or controlled by Myriad Genetics.[5][6] Myriad's business model of exclusively offering the diagnostic test led to Myriad growing from being a startup in 1994 to being a publicly traded company with 1200 employees and about $500 million in annual revenue in 2012;[7] it also led to controversy over high prices and the inability to get second opinions from other diagnostic labs, which in turn led to the landmark Association for Molecular Pathology v. Myriad Genetics lawsuit.[8]
Biallelic and homozygous inheritance of a BRCA gene leads to a severe form of Fanconi anemia, and is embryonically lethal in the majority of the cases.
Health effects
editWomen with deleterious mutations in either the BRCA1 or BRCA2 genes have a high risk of developing breast and/or ovarian cancer. Because different studies look at different populations, and because different types of mutations have somewhat different risks, the risk is best expressed as a range, rather than a single number.[9]: 89–111
Approximately 50% to 65% of women born with a deleterious mutation in BRCA1 will develop breast cancer by age 70, and 35% to 46% will develop ovarian cancer by age 70. Approximately 40% to 57% of women with a deleterious mutation in BRCA2 will develop breast cancer by age 70, and 13% to 23% will develop ovarian cancer by age 70.[9]: 89–111 [10]
Women with a breast cancer associated with a BRCA mutation have up to a 40% probability of developing a new primary breast cancer within 10 years following initial diagnosis if they did not receive tamoxifen treatment or have an oophorectomy.[4] The woman's ten-year risk for ovarian cancer is also increased by 6-12% under these conditions.[4]
Statistics for BRCA-related ovarian cancer typically encompass not only cancer of the ovaries themselves, but also peritoneal cancer and the very rare, but somewhat easier to detect, cancer of the fallopian tubes. Women with a BRCA mutation have more than 100 times the normal rate of fallopian tube cancer.[9]: 275–302 These three types of these cancers can be difficult to distinguish in their advanced stages.
Cancer onset
editBRCA-related breast cancer appears at an earlier age than sporadic breast cancer.[9]: 89–111 It has been asserted that BRCA-related breast cancer is more aggressive than normal breast cancer, however most studies in specific populations suggest little or no difference in survival rates despite seemingly worse prognostic factors.[11][12][13]
- BRCA1 is associated with triple-negative breast cancer, which does not respond to hormonal treatments and cannot be usefully treated with some drugs, such as trastuzumab. Breast cancer often appears about two decades earlier than normal.[9]: 89–111
- BRCA2 is associated primarily with post-menopausal breast cancer, although the risk of pre-menopausal breast cancer is significant. It is typically highly responsive to hormonal treatments.[9]: 89–111
BRCA-related ovarian and fallopian tube cancer is more treatable than average because it is unusually susceptible to platinum-based chemotherapy like cisplatin.[9]: 275–302 There are also FDA-approved maintenance therapies available in the form of PARP inhibitors.[14] BRCA1-related ovarian cancer appears at younger ages, but the risk for women with BRCA2 climbs markedly at or shortly after menopause.[9]: 275–302
Survival impact
edit72 / 100 | |
46 / 100 | |
11 / 100 72% of women with a BRCA1 mutation and 46% of women with a BRCA2 mutation (and no screening or medical interventions) who die before age 70 will die from breast or ovarian cancer. 11% of women in the US who die before age 70 will die from breast or ovarian cancer.[15] |
| Group | Percentage surviving to age 70 |
|---|---|
| BRCA1 mutation | |
| BRCA2 mutation | |
| Typical woman |
A 25-year-old woman with no mutation in her BRCA genes has an 84% probability to reach at least the age of 70.[15] Of those not surviving, 11% die from either breast or ovarian cancer, and 89% from other causes.
Compared to that, a woman with a high-risk BRCA1 mutation, if she had breast cancer screening but no prophylactic medical or surgical intervention, would have only 59% chance to reach age 70, twenty-five percentage points lower than normal. Of those women not surviving, 26% would die of breast cancer, 46% ovarian cancer, and 28% other causes.[15]
Women with high-risk BRCA2 mutations, with screening but with no prophylactic medical or surgical intervention, would have only 71% chance to reach age 70, thirteen percentage points lower than normal. Of those not surviving, 21% would die of breast cancer, 25% ovarian cancer and 54% other causes.[15]
The likelihood of surviving to at least age 70 can be improved by several medical interventions, notably prophylactic mastectomy and oophorectomy.[15]
Male breast cancer
editMen with a BRCA mutation have a dramatically elevated relative risk of developing breast cancer, but because the overall incidence of breast cancer in men is so low, the absolute risk is equal to or lower than the risk for women without a BRCA mutation.[9]: Ch8 Approximately 1% to 2% of men with a BRCA1 mutation will develop breast cancer by age 70. Approximately 6% of men with a BRCA2 mutation will develop breast cancer by age 70, which is approximately equal to the risk for women without a BRCA mutation. Very few men, with or without a predisposing mutation, develop breast cancer before age 50.[9]: Ch8
Approximately half of men who develop breast cancer have a mutation in a BRCA gene or in one of the other genes associated with hereditary breast–ovarian cancer syndromes.
Breast cancer in men can be treated as successfully as breast cancer in women, but men often ignore the signs and symptoms of cancer, such as a painful area or an unusual swelling, which may be no bigger than a grain of rice, until it has reached a late stage.[9]: Ch8
Unlike other men, men with a BRCA mutation, especially a BRCA2 mutation, may benefit from professional and self breast exams. Medical imaging is not usually recommended, but because male BRCA2 carriers have a risk of breast cancer that is very similar to the general female population, the standard annual mammogram program can be adapted to these high-risk men.[9]: Ch8
Other cancers
editMutations have been associated with increased risk of developing any kind of invasive cancer, including stomach cancer, pancreatic cancer, prostate cancer, and colon cancer.[16] Carriers have the normal risks of developing cancer (and other diseases) associated with increased age, smoking, alcohol consumption, poor diet, lack of exercise, and other known risk factors, plus the additional risk from the genetic mutations and an increased susceptibility to damage from ionizing radiation, including natural background radiation.[9]: 39–50
Men with BRCA mutations cannot get ovarian cancer, but they may be twice as likely as non-carriers to develop prostate cancer at a younger age.[9]: Ch8 The risk is smaller and disputed for BRCA1 carriers; up to one-third of BRCA2 mutation carriers are expected to develop prostate cancer before age 65. Prostate cancer in BRCA mutation carriers tends to appear a decade earlier than normal, and it tends to be more aggressive than normal. As a result, annual prostate screening, including a digital rectal examination, is appropriate at age 40 among known carriers, rather than age 50.[9]: Ch8
Cancer of the pancreas tends to run in families, even among BRCA families.[9]: Ch8 A BRCA1 mutation approximately doubles or triples the lifetime risk of developing pancreatic cancer; a BRCA2 mutation triples to quintuples it. Between 4% and 7% of people with pancreatic cancer have a BRCA mutation.[16] However, since pancreatic cancer is relatively rare, people with a BRCA2 mutation probably face an absolute risk of about 5%. Like ovarian cancer, it tends not to produce symptoms in the early, treatable stages. Like prostate cancer, pancreatic cancer associated with a BRCA mutation tends to appear about a decade earlier than non-hereditary cases.[16] Asymptomatic screening is invasive and may be recommended only to BRCA2 carriers who also have a family history of pancreatic cancer.[9]: Ch8
Melanoma is the most deadly skin cancer, although it is easily cured in the early stages. The normal likelihood of developing melanoma depends on race, the number of moles the person has, family history, age, sex, and how much the person has been exposed to UV radiation. BRCA2 mutation carriers have approximately double or triple the risk that they would normally have, including a higher than average risk of melanoma of the eye.[9]: Ch8 [16]
Cancer of the colon is approximately as common in both men and women in the developed world as breast cancer is among average-risk women, with about 6% of people being diagnosed with it, usually over the age of 50.[9]: Ch8 Like sporadic prostate cancer, it is a multifactorial disease, and is affected by age, diet, and similar factors. BRCA mutation carriers have a higher than average risk of this common cancer, but the risk is not as high as in some other hereditary cancers. The risk might be as high as four times normal in some BRCA1 families, and double the normal risk among BRCA2 carriers. Like pancreatic cancer, it may be that only some BRCA mutations or some BRCA families have the extra risk; unlike other BRCA-caused cancers, it does not appear at an earlier age than usual.[9]: Ch8 Normal colon cancer screening is usually recommended to BRCA mutation carriers.
Mutations in BRCA1 and BRCA2 are strongly implicated in some hematological malignancies. BRCA1 mutations are associated acute myelogenous leukemia and chronic myelogenous leukemia.[17] Mutations of BRCA2 are also found in many T-cell lymphomas and chronic lymphocytic leukemias.[17]
Childbearing
editThe dilemma of whether or not to have children may be a source of stress for women who learn of their BRCA mutations during their childbearing years.[18]
There is likely little or no effect of a BRCA gene mutation on overall fertility,[19] although women with a BRCA mutation may be more likely to have primary ovarian insufficiency.[20] [21] BRCA mutation carriers may be more likely to give birth to girls than boys,[22] however this observation has been attributed to ascertainment bias.[23][24]
If both parents are carriers of a BRCA mutation, then pre-implantation genetic diagnosis is sometimes used to prevent the birth of a child with BRCA mutations.[9]: 82–85 Inheriting two BRCA1 mutations (one from each parent) has never been reported and is believed to be a lethal birth defect. Inheriting one BRCA1 mutation and one BRCA2 mutation has been reported occasionally; the child's risk for any given type of cancer is the higher risk of the two genes (e.g., the ovarian cancer risk from BRCA1 and the pancreatic cancer risk from BRCA2). Inheriting two BRCA2 mutations produces Fanconi anemia.[9]: 82–85
Each pregnancy in genetically typical women is associated with a significant reduction in the mother's risk of developing breast cancer after age 40.[18] The younger the woman is at the time of her first birth, the more protection against breast cancer she receives.[9]: 113–142 Breastfeeding for more than one year protects against breast cancer.[9]: 113–142 Pregnancy also protects against ovarian cancer in genetically typical women.[18]
Although some studies have produced different results, women with BRCA mutations are generally not expected to receive these significant protective benefits.[9]: 113–142 [18] Current research is too limited and imprecise to permit calculation of specific risks.[18] However, the following general trends have been identified:
- For women with a BRCA1 mutation, the woman's age when she first gives birth has no association with her risk of breast cancer.[18] Childbearing provides no protection against breast cancer, unless the woman has five or more full-term pregnancies, at which point she receives only modest protection.[18] Similar to genetically typical women, pregnancy protects against ovarian cancer in BRCA1 women.[18] Breastfeeding for more than one year significantly protects against breast cancer.[18] This effect may be as high as 19% per year of breastfeeding, which is much higher than that seen among genetically typical women.[25] The effect, if any, of long-term breastfeeding on ovarian cancer is unclear.[18]
- For women with a BRCA2 mutation, each pregnancy is paradoxically associated with a statistically significant increase in the risk for breast cancer.[18] Unlike genetically typical women or women with BRCA1 mutations, breastfeeding has no effect on either cancer in women with BRCA2 mutations.[18] Limited and conflicting data suggest that, also unlike other women, pregnancy does not reduce ovarian cancer risk significantly in women with a BRCA2 mutation and might increase it.[18]
Biallelic and homozygous inheritance
editReports of patients biallelic or homozygous for a deleterious BRCA allele conferring a greatly increased risk of breast cancer are rare. This is because deleterious BRCA alleles are lethal alleles; this condition is embryonically lethal in the majority of the cases.[26] For live cases, inheriting both mutations lead to a grave prognosis, characterized by Wilms tumors, leukemias, and early-onset brain malignancies.[27]
Genetics
editBoth BRCA genes are tumor suppressor genes that produce proteins that are used by the cell in an enzymatic pathway that makes very precise, perfectly matched repairs to DNA molecules that have double-stranded breaks.[9]: 39–50 [28] The pathway requires proteins produced by several other genes, including CHK2, FANCD2 and ATM.[16] Harmful mutations in any of these genes disable the gene or the protein that it produces.
The cancer risk caused by BRCA1 and BRCA2 mutations are inherited in a dominant fashion even though usually only one mutated allele is directly inherited.[29] This is because people with the mutation are likely to acquire a second mutation, leading to dominant expression of the cancer. A mutated BRCA gene can be inherited from either parent. Because they are inherited from the parents, they are classified as hereditary or germline mutations rather than acquired or somatic mutations. Cancer caused by a mutated gene inherited from an individual's parents is a hereditary cancer rather than a sporadic cancer.
Because humans have a diploid genome, each cell has two copies of the gene (one from each biological parent). Typically only one copy contains a disabling, inherited mutation, so the affected person is heterozygous for the mutation. If the functional copy is harmed, however, then the cell is forced to use alternate DNA repair mechanisms, which are more error-prone. The loss of the functional copy is called loss of heterozygosity (LOH).[30] Any resulting errors in DNA repair may result in cell death or a cancerous transformation of the cell.[9]: 39–50
There are many variations in BRCA genes, and not all changes confer the same risks. [9]: 39–50 Some variants are harmless; others are known to be very harmful. Some single nucleotide polymorphisms may confer only a small risk, or may only confer risk in the presence of other mutations or under certain circumstances. In other cases, whether the variant is harmful is unknown. Variants are classified as follows:[9]: 39–50 : 109
- Deleterious mutation: The change is proven to cause significant risks. Often, these are frameshift mutations that prevent the cell from producing more than the first part of the necessary protein.
- Suspected deleterious: While nothing is proven, the variation is currently believed to be harmful.
- Variant of uncertain significance (VUS): Whether the change has any effect is uncertain. This is a common test result, and most variations began in this category. As more evidence is acquired, these are re-classified.
- Variant, favor polymorphism: While nothing is proven, the variation is currently believed to be harmless.
- Benign polymorphism: The change is classified as harmless. These may be reported as "no mutation".
Deleterious mutations have high, but not complete, genetic penetrance, which means that people with the mutation have a high risk of developing disease as a result, but that some people will not develop cancer despite carrying a harmful mutation.
Diagnosis
editGenetic counseling is recommended in women whose personal or family health history suggests a greater than average likelihood of a mutation.[31] The purpose of genetic counseling is to educate the person about the likelihood of a positive result, the risks and benefits of being tested, the limitations of the tests, the practical meaning of the results, and the risk-reducing actions that could be taken if the results are positive. They are also trained to support people through any emotional reactions and to be a neutral person who helps the client make his or her own decision in an informed consent model, without pushing the client to do what the counselor might do. Because the knowledge of a mutation can produce substantial anxiety, some people choose not to be tested or to postpone testing until a later date.[9]: 51–74
Relative indications for testing for a mutation in BRCA1 or BRCA2 for newly diagnosed or family members include a family history among 1st (FDR), 2nd (SDR), or 3rd(TDR) degree relatives usually on the same side of the family but not limited:[1][32]
- A known mutation (BRCA1 or BRCA2) in a cancer susceptibility gene within the family
- Women affected by any breast cancer diagnosed under the age of 30[33]
- Women affected by triple negative breast cancer (TNBC) (estrogen receptor negative, progesterone receptor negative, and HER2/neu negative) under the age of 50
- Two relatives (FDR/SDR) diagnosed under the age of 45
- Three relatives (FDR/SDR) diagnosed with average age of 50 or less
- Four relatives at any ages
- Ovarian cancer with either an additional diagnosed relative or a relative with male breast cancer
- A single family member with both breast and ovarian cancer
- Male breast cancer
- Pancreatic cancer with breast or ovarian cancer in the same individual or on the same side of the family
- Ashkenazi Jewish, Filipino, or Polish ancestry with one FDR family member affected by breast or ovarian cancer at any age[34]
Testing young children is considered medically unethical because the test results would not change the way the child's health is cared for.[9]: 82–85
Test procedure
editTwo types of tests are available.[9]: 51–74 Both commonly use a blood sample, although testing can be done on saliva. The quickest, simplest, and lowest cost test uses positive test results from a blood relative and checks only for the single mutation that is known to be present in the family. If no relative has previously disclosed positive test results, then a full test that checks the entire sequence of both BRCA1 and BRCA2 can be performed. In some cases, because of the founder effect, Jewish ethnicity can be used to narrow the testing to quickly check for the three most common mutations seen among Ashkenazi Jews.[9]: 51–74
Testing is commonly covered by health insurance and public healthcare programs for people at high risk for having a mutation, and not covered for people at low risk.[9]: 51–74 The purpose of limiting the testing to high-risk people is to increase the likelihood that the person will receive a meaningful, actionable result from the test, rather than identifying a variant of unknown significance (VUS). In Canada, people who demonstrate their high-risk status by meeting specified guidelines are referred initially to a specialized program for hereditary cancers, and, if they choose to be tested, the cost of the test is fully covered. In the US in 2010, single-site testing had a retail cost of US$400 to $500, and full-length analysis cost about $3,000 per gene, and the costs were commonly covered by private health insurance for people deemed to be at high risk.
The test is ordered by a physician, usually an oncologist, and the results are always returned to the physician, rather than directly to the patient. How quickly results are returned depends on the test—single-site analysis requires less lab time—and on the infrastructure in place. In the US, test results are commonly returned within one to several weeks; in Canada, patients commonly wait for eight to ten months for test results.[9]: 51–74
Test interpretation
editA positive test result for a known deleterious mutation is proof of a predisposition, although it does not guarantee that the person will develop any type of cancer. A negative test result, if a specific mutation is known to be present in the family, shows that the person does not have a BRCA-related predisposition for cancer, although it does not guarantee that the person will not develop a non-hereditary case of cancer. By itself, a negative test result does not mean that the patient has no hereditary predisposition for breast or ovarian cancer. The family may have some other genetic predisposition for cancer, involving some other gene.[9]: 89–111
Cancer prevention
editA variety of screening options and interventions are available to manage BRCA-related cancer risks. Screenings are adjusted to individual and familial risk factors.[35]
As these screening methods do not prevent cancer, but merely attempt to catch it early, numerous methods of prevention are sometimes practiced, with varying results.[9]: 175–207
Screening
editAn intensive cancer screening regimen is usually advised for women with deleterious or suspected deleterious BRCA mutations in order to detect new cancers as early as possible. A typical recommendation includes frequent breast cancer screening as well as tests to detect ovarian cancer.[9]: 175–207
Breast imaging studies usually include a breast MRI (magnetic resonance imaging) once a year, beginning between ages 20 and 30, depending on the age at which any relatives were diagnosed with breast cancer. Mammograms are typically used only at advanced age as there is reason to believe that BRCA carriers are more susceptible to breast cancer induction by X-ray damage than general population.[36]
Alternatives include breast ultrasonography, CT scans, PET scans, scintimammography, elastography, thermography, ductal lavage, and experimental screening protocols, some of which hope to identify biomarkers for breast cancer (molecules that appear in the blood when breast cancer begins).[9]: 175–207
Ovarian cancer screening usually involves ultrasonography of the pelvic region, typically twice a year.[9]: 175–207 Women may also use a blood test for CA-125 and clinical pelvic exams. The blood test has relatively poor sensitivity and specificity for ovarian cancer.[9]: 175–207 [37]
In both breast and ovarian screening, areas of tissue that look suspicious are investigated with either more imaging, possibly using a different type of imaging or after a delay, or with biopsies of the suspicious areas.
Medication
editBirth control pills are associated with substantially lower risk of ovarian cancer in women with BRCA mutations.[38][39] A 2013 meta-analysis found that oral contraceptive use was associated with a 42% reduction of the relative risk of ovarian cancer, the association was similar for BRCA1 and BRCA2 mutations. Use of oral contraceptives was not significantly associated with breast cancer risk although a small increase of risk that did not reach statistical significance was observed.[38][39] A 2011 meta-analysis found that OC use was associated with a 43% relative reduction in risk of ovarian cancer in women with BRCA mutations, while data on the risk of breast cancer in BRCA mutation carriers with oral contraceptive use were heterogeneous and results were inconsistent.[40]
Selective estrogen receptor modulators, specifically tamoxifen, have been found to reduce breast cancer risk in women with BRCA mutations who do not have their breast removed.[9]: 113–142 It is effective as for primary prevention (preventing the first case of breast cancer) in women with BRCA2 mutations, but not BRCA1 mutations, and for secondary prevention (preventing a second, independent breast cancer) in both groups of women. Taking tamoxifen for five years has been found to halve the breast cancer risk in women who have a high risk of breast cancer for any reason, but potentially serious adverse effects like cataracts, blood clots, and endometrial cancer, along with quality of life issues like hot flashes, result in some women discontinuing its use and some physicians limiting its use to women with atypical growths in the breasts. Tamoxifen is contraindicated for women who are most likely to be harmed by the common complications. Raloxifene (Evista), which has a reduced risk of side effects, is used as an alternative, but it has not been studied in BRCA mutation carriers specifically. Tamoxifen use can be combined with oophorectomy for even greater reduction of breast cancer risk, particularly in women with BRCA2 mutations.[9]: 113–142
Aromatase inhibitors are medications that prevent estrogen production in the adrenal glands and adipose tissue. They have fewer side effects than selective estrogen receptor modulators like tamoxifen, but do not work in premenopausal women, because they do not prevent the ovaries from producing estrogen.[9]: 113–142
Surgery
editSeveral type of preventive surgeries are known to substantially reduce cancer risk for women with high-risk BRCA mutations.[41] The surgeries may be used alone, in combination with each other, or in combination with non-surgical interventions to reduce the risk of breast and ovarian cancer. Surgeries such as mastectomy and oophorectomy do not eliminate the chance of breast cancer; cases have reportedly emerged despite these procedures.[42]
- Tubal ligation is the least invasive of these surgeries and appears to reduce ovarian cancer risk for BRCA1 carriers by over 60%. Salpingectomy is another option which is more invasive than tubal ligation and may result in additional risk reduction. Both of these can be performed anytime after childbearing is complete.[41] Unlike other prophylactic surgeries, these two surgeries do not reduce the risk of breast cancer.[43]
- Prophylactic (preventive) mastectomy is associated with small risks and a large drop in breast cancer risk.
- Prophylactic salpingo-oophorectomy (removal of the ovaries and fallopian tubes) results in a very large reduction in ovarian cancer risk, and a large reduction in breast cancer risk if performed before natural menopause. However, it also comes with the risk of substantial adverse effects if performed at a young age.
- Hysterectomy has no direct effect on BRCA-related cancers, but it enables the women to use some medications that reduce breast cancer risk (such as tamoxifen) with the risk of uterine cancer and to use fewer hormones to manage the adverse effects of a prophylactic oophorectomy.
Whether and when to perform which preventive surgeries is a complex personal decision. Current medical knowledge offers some guidance about the risks and benefits. Even carriers of the same mutation or from the same family may have substantially different risks for the kind and severity of cancer they are likely to get, as well as the age at which they may get them. Different people also have different values. They may choose to focus on total cancer prevention, psychological benefits, current quality of life, or overall survival. The possible impact of future medical developments in treatment or prognosis may also be of some importance for very young women and family planning. The decision is individualized and is usually based on many factors, such as earliest occurrence of BRCA-related cancer in close relatives.
An increasing number women who test positive for faulty BRCA1 or BRCA2 genes choose to have risk-reducing surgery. At the same time the average waiting time for undergoing the procedure is two-years which is much longer than recommended.[44][45]
The protective effect of prophylactic surgery is greater when done at young age; however, oophorectomy also has adverse effects that are greatest when done long before natural menopause. For this reason, oophorectomy is mostly recommended after age 35 or 40, assuming childbearing is complete. The risk of ovarian cancer is low before this age, and the negative effects of oophorectomy are less serious as the woman nears natural menopause.[15][46]
- For carriers of high-risk BRCA1 mutations, prophylactic oophorectomy around age 40 reduces the risk of ovarian and breast cancer and provides a substantial long-term survival advantage. Having this surgery at a very young age provides little or no additional survival advantage, but it does increase the adverse effects from the surgery. Compared to no intervention, having this surgery around age 40 increases the woman's chance of reaching age 70 by fifteen percentage points, from 59% to 74%. Adding prophylactic mastectomy increases the expected survival by several more percentage points.
- For carriers of high-risk BRCA2 mutations, oophorectomy around age 40 has a smaller effect. The surgery increases the woman's chance of reaching age 70 by only five percentage points, from 75% to 80%. When only preventive mastectomy is done at age 40 instead, the improvement is similar, with the expected chance rising from 75% to 79%. Doing both surgeries together around age 40 is expected to improve the woman's chance of reaching age 70 from 75% to 82%
For comparison, women in the general population have an 84% chance of living to age 70.
Research has looked into the effects of risk-reducing surgery on the psychological and social wellbeing of women with a BRCA mutation.[47] Due to limited evidence, a 2019 meta analysis was unable to draw conclusions on whether interventions can help with the psychological effects of surgery in female BRCA carriers. More research is needed to conclude how best to support women who choose surgery.[47]
Mastectomy
editIn a woman who has not developed breast cancer, removing the breasts may reduce her risk of ever being diagnosed with breast cancer by 90%, to a level that is approximately half the average woman's risk.[9]: 209–244
Bilateral mastectomy is the removal of both breasts by a breast surgeon.[9]: 209–244 The modified radical mastectomy is only used in women diagnosed with invasive breast cancer. Techniques for prophylactic mastectomies include:[9]: 209–244
- Simple mastectomy, which is recommended for women not having breast reconstruction, leaves the least amount of breast tissue in the body and therefore achieves the greatest risk reduction. In addition to prophylactic use, it is also used by women who have been diagnosed with earlier stages of cancer.
- Skin-sparing mastectomy removes the tissue of the breast, nipple, and areola, but leave the "excess" skin in place for reconstruction. It has less visible scar tissue than a simple mastectomy.
- Nipple-sparing mastectomy removes the breast tissue, but leaves the nipple and the areola intact for a more natural appearance.
- Subcutaneous mastectomy removes the breast tissue, but leaves the nipple and areola intact. The scars are hidden in the inframammary fold under the breast.
- Areola-sparing mastectomy removes the breast tissue and the nipple, but not the areola.
- Nerve-sparing mastectomy is an effort to maintain the nerves that provide sensation to the skin over the breasts. Breasts that have undergone any of these surgeries have much less tactile sensation than natural breasts. Nerve-sparing techniques are an effort to retain some feeling in the breasts, with limited and often only partial success.[9]: 209–244
Which technique is used is determined by the existence of any cancer and overall health, as well as by the woman's desire, if any, for breast reconstruction surgery for aesthetic purposes.[9]: 209–244 Women who choose a flat-chested appearance or use external breast prostheses typically choose simple mastectomy, with its greater risk reduction.[9]: 209–244
Breast reconstruction is usually done by a plastic surgeon, and may be started as part of the same multi-hour surgery that removes the breasts. Multiple techniques for reconstruction have been used, with different locations and amounts of scarring. Some techniques use tissue from another part of the body, such as fat tissue from the lower abdomen or occasionally muscles from other parts of the torso. Others use breast implants, possibly preceded by tissue expanders, to provide volume. Some reconstruction techniques require multiple surgeries. Afterwards, some women have tattoos added to simulate breast areolas or have the skin reshaped to form a nipple.[9]: 209–244
Salpingo-oophorectomy
editOophorectomy (surgical removal of the ovaries) and salpingectomy (surgical removal of the fallopian tubes) are strongly recommended to women with BRCA mutations.[9]: 275–302 Salpingo-oophorectomy is the single most effective method of preventing ovarian and fallopian tube cancer in women with a BRCA mutation. However, a small risk of primary peritoneal cancer remains, at least among women with BRCA1 mutations, since the peritoneal lining is the same type of cells as parts of the ovary. This risk is estimated to produce about five cases of peritoneal cancer per 100 women with harmful BRCA1 mutations in the 20 years after the surgery.[9]: 275–302
BRCA2 related ovarian cancer tends to present in perimenopausal or menopausal women, so salpingo-oophorectomy is recommended between ages 45 and 50.[9]: 275–302
The surgery is often done in conjunction with a hysterectomy (surgical removal of the uterus) and sometimes a cervicectomy (surgical removal of the cervix), especially in women who want to take tamoxifen, which is known to cause uterine cancer, or who have uterine fibroids.[9]: 275–302 Multiple styles of surgery are available, including laparoscopic (keyhole) surgery. Because about 5% of women with a BRCA mutation have undetected ovarian cancer at the time of their planned surgery, the surgery should be treated as if it were a removal of a known cancer.[9]: 275–302
Salpingo-oophorectomy makes the woman sterile (unable to bear children). Infertility services can be used to preserve her eggs, if wanted. However, as the benefits to the surgery are greatest close to menopause, most women simply postpone the surgery until they have already borne as many children as they choose to.[9]: 275–302
The surgery also artificially induces menopause, which causes hot flashes, sleep disturbances, mood swings, vaginal dryness, sexual difficulties, difficulty with word recall, and other medical signs and symptoms. The side effects range from mild to severe; most can be treated at least partially. Many women with a BRCA take hormone replacement therapy to reduce these effects: estrogen-progesterone combinations for women who have a uterus, and unopposed estrogen for women whose uterus was removed. Estrogen can cause breast cancer, but as the amount of estrogen taken is less than the amount produced by the now-removed ovaries, the net risk is usually judged to be acceptable.[9]: 303–317
Some sources assume that oophorectomy before age 50 doubles the risk of cardiovascular disease and increases risk of hip fractures caused by osteoporosis in the relevant population.[15]
Non-medical choices
editGiven the high risks and the low benefit of lifestyle choices in BRCA mutation carriers, no lifestyle choices provide sufficient protection.[9]: 113–142
Having her first child at a younger age, having more children than average, and breastfeeding for more than one year decreases the risk of breast cancer for an average-risk woman.[9]: 113–142 Studies about this effect among BRCA mutation carriers have produced conflicting results, but generally speaking, having children is believed to provide little or no protection against breast cancer for women with BRCA1 mutations, and to paradoxically increase the risk of breast cancer for women with BRCA2 mutations.[9]: 113–142 [18]
Being physically active and maintaining a healthy body weight prevents breast and other cancers in the general population, as well as preventing heart disease and other medical conditions. Among women with a BRCA mutation, being physically active and having had a healthy body weight as an adolescent has no effect on ovarian cancer and delays, but does not entirely prevent, breast cancer after menopause.[9]: 113–142 [48] In some studies, only significant, strenuous exercise produced any benefit.[9]: 113–142 Obesity and weight gain as an adult are associated with breast cancer diagnoses.[9]: 113–142
Studies on specific foods, diets, or dietary supplements have generally produced conflicting information or, in the case of dietary fat, soy consumption, and drinking green tea, have only been conducted in average-risk women.[9]: 113–142 The only dietary intervention that is generally accepted as preventing breast cancer in BRCA mutation carriers is minimizing consumption of alcoholic beverages. Consuming more than one alcoholic drink per day is strongly associated with a higher risk of developing breast cancer, and carriers are usually encouraged to consume no more than one alcoholic drink per day, and no more than four total in a week.[9]: 113–142
In a study conducted with Ashkenazi Jewish women, it was observed that mutation carriers born before 1940 have a much lower risk of being diagnosed with breast cancer by age 50 than those born after 1940; this was also observed in the non-carrier population.[48] The reasons for the difference is unknown. Unlike the general population, age at menarche and age at menopause has no effect on breast cancer risk for BRCA mutation carriers.[9]: 113–142
Evolutionary advantage
editStudies have shown that BRCA1 mutations are not random, but under adaptive selection, indicating that although BRCA1 mutations are linked to breast cancer, the mutations likely have a beneficial effect as well.[49]
Patents
editA patent application for the isolated BRCA1 gene and cancer-cancer promoting mutations discussed above, as well as methods to diagnose the likelihood of getting breast cancer, was filed by the University of Utah, National Institute of Environmental Health Sciences (NIEHS) and Myriad Genetics in 1994;[5] over the next year, Myriad, in collaboration with investigators from Endo Recherche, Inc., HSC Research & Development Limited Partnership, and University of Pennsylvania, isolated and sequenced the BRCA2 gene and identified key mutations, and the first BRCA2 patent was filed in the US by Myriad and other institutions in 1995.[6] Myriad is the exclusive licensee of these patents and has enforced them in the US against clinical diagnostic labs.[8] This business model led to Myriad growing being a startup in 1994 to being a publicly traded company with 1200 employees and about $500M in annual revenue in 2012;[7] it also led to controversy over high prices and the inability to get second opinions from other diagnostic labs, which in turn led to the landmark Association for Molecular Pathology v. Myriad Genetics lawsuit.[8][50] The patents began to expire in 2014.
According to an article published in the journal, Genetic Medicine, in 2010, "The patent story outside the United States is more complicated.... For example, patents have been obtained but the patents are being ignored by provincial health systems in Canada. In Australia and the UK, Myriad's licensee permitted use by health systems, but announced a change of plans in August 2008. ... Only a single mutation has been patented in Myriad's lone European-wide patent, although some patents remain under review of an opposition proceeding. In effect, the United States is the only jurisdiction where Myriad's strong patent position has conferred sole-provider status."[51][52] Peter Meldrum, CEO of Myriad Genetics, has acknowledged that Myriad has "other competitive advantages that may make such [patent] enforcement unnecessary" in Europe.[53]
Legal decisions surrounding the BRCA1 and BRCA2 patents will affect the field of genetic testing in general.[54] In June 2013, in Association for Molecular Pathology v. Myriad Genetics (No. 12-398), the US Supreme Court unanimously ruled that, "A naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated," invalidating Myriad's patents on the BRCA1 and BRCA2 genes. However, the Court also held that manipulation of a gene to create something not found in nature could still be eligible for patent protection.[55]
See also
editReferences
edit- ^ a b Petrucelli N, Daly MB, Pal T (September 1998). "BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer". In Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, Gripp KW, Amemiya A, editors (eds.). GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle. PMID 20301425.
- ^ Yan H (14 May 2013). "What's the gene that led to Angelina Jolie's double mastectomy?". Health. CNN.
- ^ "BRCA1 and BRCA2: Cancer Risk and Genetic Testing". National Cancer Institute. 29 May 2009.
- ^ a b c Weitzel JN, Lagos VI, Cullinane CA, Gambol PJ, Culver JO, Blazer KR, et al. (June 2007). "Limited family structure and BRCA gene mutation status in single cases of breast cancer". JAMA. 297 (23): 2587–2595. doi:10.1001/jama.297.23.2587. PMID 17579227.
- ^ a b US5747282
- ^ a b US5837492
- ^ a b Myriad Investor Page—see "Myriad at a glance" Archived 18 October 2012 at the Wayback Machine accessed October 2012
- ^ a b c Schwartz J (12 May 2009). "Cancer Patients Challenge the Patenting of a Gene". The New York Times.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn bo Morris JL, Gordon OK (2010). Positive Results: Making the Best Decisions When You're at High Risk for Breast or Ovarian Cancer. Amherst, N.Y.: Prometheus Books. ISBN 978-1-59102-776-8.
- ^ Chen S, Parmigiani G (April 2007). "Meta-analysis of BRCA1 and BRCA2 penetrance". Journal of Clinical Oncology. 25 (11): 1329–1333. doi:10.1200/JCO.2006.09.1066. PMC 2267287. PMID 17416853.
- ^ Veronesi A, de Giacomi C, Magri MD, Lombardi D, Zanetti M, Scuderi C, et al. (July 2005). "Familial breast cancer: characteristics and outcome of BRCA 1-2 positive and negative cases". BMC Cancer. 5: 70. doi:10.1186/1471-2407-5-70. PMC 1184063. PMID 15996267.
- ^ Budroni M, Cesaraccio R, Coviello V, Sechi O, Pirino D, Cossu A, et al. (February 2009). "Role of BRCA2 mutation status on overall survival among breast cancer patients from Sardinia". BMC Cancer. 9: 62. doi:10.1186/1471-2407-9-62. PMC 2653541. PMID 19232099.
- ^ Verhoog LC, Brekelmans CT, Seynaeve C, van den Bosch LM, Dahmen G, van Geel AN, et al. (January 1998). "Survival and tumour characteristics of breast-cancer patients with germline mutations of BRCA1". Lancet. 351 (9099): 316–321. doi:10.1016/S0140-6736(97)07065-7. PMID 9652611. S2CID 38655517.
- ^ O'Malley DM, Krivak TC, Kabil N, Munley J, Moore KN (July 2023). "PARP Inhibitors in Ovarian Cancer: A Review". Targeted Oncology. 18 (4): 471–503. doi:10.1007/s11523-023-00970-w. PMC 10344972. PMID 37268756.
- ^ a b c d e f g Kurian AW, Sigal BM, Plevritis SK (January 2010). "Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers". Journal of Clinical Oncology. 28 (2): 222–231. doi:10.1200/JCO.2009.22.7991. PMC 2815712. PMID 19996031.
- ^ a b c d e Friedenson B (June 2005). "BRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian". MedGenMed. 7 (2): 60. PMC 1681605. PMID 16369438.
- ^ a b Friedenson B (August 2007). "The BRCA1/2 pathway prevents hematologic cancers in addition to breast and ovarian cancers". BMC Cancer. 7: 152. doi:10.1186/1471-2407-7-152. PMC 1959234. PMID 17683622.
- ^ a b c d e f g h i j k l m n Fishman A (October 2010). "The effects of parity, breastfeeding, and infertility treatment on the risk of hereditary breast and ovarian cancer: a review". International Journal of Gynecological Cancer. 20 (11 Suppl 2): S31–S33. doi:10.1111/IGC.0b013e3181f60d4d. PMID 20975359. S2CID 42755864.
- ^ Pal T, Keefe D, Sun P, Narod SA (April 2010). "Fertility in women with BRCA mutations: a case-control study". Fertility and Sterility. 93 (6): 1805–1808. doi:10.1016/j.fertnstert.2008.12.052. PMID 19200971.
- ^ Broer SL, Broekmans FJ, Laven JS, Fauser BC (2014). "Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications". Human Reproduction Update. 20 (5): 688–701. doi:10.1093/humupd/dmu020. PMID 24821925.
- ^ Oktay K, Kim JY, Barad D, Babayev SN (January 2010). "Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks". Journal of Clinical Oncology. 28 (2): 240–244. doi:10.1200/JCO.2009.24.2057. PMC 3040011. PMID 19996028.
- ^ Moslehi R, Singh R, Lessner L, Friedman JM (March–April 2010). "Impact of BRCA mutations on female fertility and offspring sex ratio". American Journal of Human Biology. 22 (2): 201–205. doi:10.1002/ajhb.20978. PMC 3739697. PMID 19642207.
- ^ Balmaña J, Díez O, Campos B, Majewski M, Sanz J, Alonso C, et al. (August 2005). "Sex ratio distortion in offspring of families with BRCA1 or BRCA2 mutant alleles: an ascertainment bias phenomenon?". Breast Cancer Research and Treatment. 92 (3): 273–277. doi:10.1007/s10549-005-3377-x. PMID 16155798. S2CID 21830848.
- ^ Agnese DM (March 2006). "Battle of the BRCA1/BRCA2 (offspring) sex ratios: truth or consequences". Journal of Medical Genetics. 43 (3): 201–202. doi:10.1136/jmg.2004.028977. PMC 2563236. PMID 16033919.
- ^ Kotsopoulos J, Lubinski J, Salmena L, Lynch HT, Kim-Sing C, Foulkes WD, et al. (March 2012). "Breastfeeding and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers". Breast Cancer Research. 14 (2): R42. doi:10.1186/bcr3138. PMC 3446376. PMID 22405187.
- ^ Sawyer SL, Tian L, Kähkönen M, Schwartzentruber J, Kircher M, Majewski J, et al. (February 2015). "Biallelic mutations in BRCA1 cause a new Fanconi anemia subtype". Cancer Discovery. 5 (2): 135–142. doi:10.1158/2159-8290.CD-14-1156. PMC 4320660. PMID 25472942.
- ^ Maxwell KN, Patel V, Nead KT, Merrill S, Clark D, Jiang Q, et al. (January 2023). "Fanconi anemia caused by biallelic inactivation of BRCA2 can present with an atypical cancer phenotype in adulthood". Clinical Genetics. 103 (1): 119–124. doi:10.1111/cge.14231. PMC 9742260. PMID 36089892.
- ^ Yoshida K, Miki Y (November 2004). "Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage". Cancer Science. 95 (11): 866–871. doi:10.1111/j.1349-7006.2004.tb02195.x. PMC 11159131. PMID 15546503. S2CID 24297965.
- ^ Cui J, Antoniou AC, Dite GS, Southey MC, Venter DJ, Easton DF, et al. (February 2001). "After BRCA1 and BRCA2-what next? Multifactorial segregation analyses of three-generation, population-based Australian families affected by female breast cancer". American Journal of Human Genetics. 68 (2): 420–431. doi:10.1086/318187. PMC 1235275. PMID 11133358.
- ^ Greenberg RA (September 2006). "BRCA mutations and childhood cancer". Cancer Biology & Therapy. 5 (9): 1103–1104. doi:10.4161/cbt.5.9.3370. PMC 2703724. PMID 17012842.
- ^ Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, et al. (August 2019). "Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: US Preventive Services Task Force Recommendation Statement". JAMA. 322 (7): 652–665. doi:10.1001/jama.2019.10987. PMID 31429903.
- ^ Frank TS (2002). "Hereditary Risk of Breast and Ovarian Cancer: BRCA1 and BRCA2". Encyclopedia of Cancer: 381–385. doi:10.1016/b0-12-227555-1/00090-3. ISBN 978-0-12-227555-5.
- ^ Rosenberg SM, Ruddy KJ, Tamimi RM, Gelber S, Schapira L, Come S, et al. (June 2016). "BRCA1 and BRCA2 Mutation Testing in Young Women With Breast Cancer". JAMA Oncology. 2 (6): 730–736. doi:10.1001/jamaoncol.2015.5941. PMC 5002892. PMID 26867710.
- ^ "Referral guidelines - cancer genetics". www.gosh.nhs.uk. Retrieved 21 February 2016.
- ^ Kukafka R, Pan S, Silverman T, Zhang T, Chung WK, Terry MB, Fleck E, Younge RG, Trivedi MS, McGuinness JE, He T, Dimond J, Crew KD (18 July 2022). "Patient and Clinician Decision Support to Increase Genetic Counseling for Hereditary Breast and Ovarian Cancer Syndrome in Primary Care". JAMA Network Open. 5 (7). American Medical Association (AMA): e2222092. doi:10.1001/jamanetworkopen.2022.22092. ISSN 2574-3805. PMC 9294997. PMID 35849397.
- ^ Pijpe A, Andrieu N, Easton DF, Kesminiene A, Cardis E, Noguès C, et al. (September 2012). "Exposure to diagnostic radiation and risk of breast cancer among carriers of BRCA1/2 mutations: retrospective cohort study (GENE-RAD-RISK)". BMJ. 345: e5660. doi:10.1136/bmj.e5660. PMC 3435441. PMID 22956590.
- ^ Lynch HT, Casey MJ, Snyder CL, Bewtra C, Lynch JF, Butts M, Godwin AK (April 2009). "Hereditary ovarian carcinoma: heterogeneity, molecular genetics, pathology, and management". Molecular Oncology. 3 (2): 97–137. doi:10.1016/j.molonc.2009.02.004. PMC 2778287. PMID 19383374.
- ^ a b Moorman PG, Havrilesky LJ, Gierisch JM, Coeytaux RR, Lowery WJ, Peragallo Urrutia R, et al. (November 2013). "Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis". Journal of Clinical Oncology. 31 (33): 4188–4198. doi:10.1200/JCO.2013.48.9021. PMID 24145348.
- ^ a b Iodice S, Barile M, Rotmensz N, Feroce I, Bonanni B, Radice P, et al. (August 2010). "Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis". European Journal of Cancer. 46 (12): 2275–2284. doi:10.1016/j.ejca.2010.04.018. PMID 20537530.
Lu KH, Berchuck A, Kauff ND (2013). "Hereditary gynecologic cancers". In Barakat RD, Berchuck A, Markman M, Randall ME (eds.). Principles and practice of gynecologic oncology (6th ed.). Philadelphia: Lippincott Williams & Wilkins. ISBN 978-1-4698-3148-0.
Isaacs C, Fletcher SW, Peskin BN (May 2013). Hayes DF, Duda RB (eds.). "Management of hereditary breast and ovarian cancer syndrome and patients with BRCA mutations". Waltham, Mass.: UpToDate. Retrieved 13 June 2013.
PDQ Cancer Genetics Editorial Board (7 June 2013). "PDQ Genetics of breast and ovarian cancer (Health professional version)". Bethesda, Md.: National Cancer Institute. Retrieved 13 June 2013. - ^ Cibula D, Zikan M, Dusek L, Majek O (August 2011). "Oral contraceptives and risk of ovarian and breast cancers in BRCA mutation carriers: a meta-analysis". Expert Review of Anticancer Therapy. 11 (8): 1197–1207. doi:10.1586/era.11.38. PMID 21916573. S2CID 31012428.
- ^ a b Pruthi S, Gostout BS, Lindor NM (December 2010). "Identification and Management of Women With BRCA Mutations or Hereditary Predisposition for Breast and Ovarian Cancer". Mayo Clinic Proceedings. 85 (12): 1111–1120. doi:10.4065/mcp.2010.0414. PMC 2996153. PMID 21123638.
- ^ Burke W, Daly M, Garber J, Botkin J, Kahn MJ, Lynch P, et al. (March 1997). "Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. Cancer Genetics Studies Consortium". JAMA. 277 (12): 997–1003. doi:10.1001/jama.1997.03540360065034. PMID 9091675.
- ^ Kwon JS, Tinker A, Pansegrau G, McAlpine J, Housty M, McCullum M, Gilks CB (January 2013). "Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers". Obstetrics and Gynecology. 121 (1): 14–24. doi:10.1097/AOG.0b013e3182783c2f. PMID 23232752. S2CID 41081248.
- ^ "Earlier decisions on breast and ovarian surgery reduce cancer in women at high risk". NIHR Evidence (Plain English summary). National Institute for Health and Care Research. 7 December 2021. doi:10.3310/alert_48318. S2CID 263487127.
- ^ Marcinkute R, Woodward ER, Gandhi A, Howell S, Crosbie EJ, Wissely J, et al. (February 2022). "Uptake and efficacy of bilateral risk reducing surgery in unaffected female BRCA1 and BRCA2 carriers". Journal of Medical Genetics. 59 (2): 133–140. doi:10.1136/jmedgenet-2020-107356. PMID 33568438. S2CID 231876899.
- ^ Stadler ZK, Kauff ND (January 2010). "Weighing options for cancer risk reduction in carriers of BRCA1 and BRCA2 mutations". Journal of Clinical Oncology. 28 (2): 189–191. doi:10.1200/JCO.2009.25.6875. PMID 19996025.
- ^ a b Jeffers L, Reid J, Fitzsimons D, Morrison PJ, Dempster M (October 2019). "Interventions to improve psychosocial well-being in female BRCA-mutation carriers following risk-reducing surgery". The Cochrane Database of Systematic Reviews. 2019 (10): CD012894. doi:10.1002/14651858.cd012894.pub2. PMC 6784162. PMID 31595976.
- ^ a b King MC, Marks JH, Mandell JB (October 2003). "Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2". Science. 302 (5645): 643–646. Bibcode:2003Sci...302..643K. doi:10.1126/science.1088759. PMID 14576434. S2CID 33441900.
- ^ Huttley GA, Easteal S, Southey MC, Tesoriero A, Giles GG, McCredie MR, et al. (August 2000). "Adaptive evolution of the tumour suppressor BRCA1 in humans and chimpanzees. Australian Breast Cancer Family Study". Nature Genetics. 25 (4): 410–413. doi:10.1038/78092. PMID 10932184. S2CID 10004449.
- ^ "ACLU sues over patents on breast cancer genes". CNN. Archived from the original on 15 May 2009. Retrieved 14 May 2009.
- ^ Cook-Deegan R, DeRienzo C, Carbone J, Chandrasekharan S, Heaney C, Conover C (April 2010). "Impact of gene patents and licensing practices on access to genetic testing for inherited susceptibility to cancer: comparing breast and ovarian cancers with colon cancers". Genetics in Medicine. 12 (4 Suppl): S15–S38. doi:10.1097/GIM.0b013e3181d5a67b. PMC 3047448. PMID 20393305.
- ^ Benowitz S (January 2003). "European groups oppose Myriad's latest patent on BRCA1". Journal of the National Cancer Institute. 95 (1): 8–9. doi:10.1093/jnci/95.1.8. PMID 12509391.
- ^ Conley J, Vorhous D, Cook-Deegan J (1 March 2011). "How Will Myriad Respond to the Next Generation of BRCA Testing?". Robinson, Bradshaw, and Hinson. Retrieved 9 December 2012.
- ^ "Genetics and Patenting". Human Genome Project Information. U.S. Department of Energy Genome Programs. 7 July 2010.
- ^ Liptak A (13 June 2013). "Supreme Court Rules Human Genes May Not Be Patented". The New York Times. Retrieved 13 June 2013.
External links
edit- BOADICEA Archived 22 November 2021 at the Wayback Machine, a risk estimator tool for familial breast and ovarian cancer
- BRCA1 and BRCA2 at Lab Tests Online
- BRCA Exchange, large database of BRCA1 and BRCA2 variants with pathogenicity classifications.