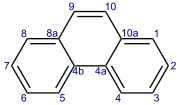
Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) with formula C14H10, consisting of three fused benzene rings. It is a colorless, crystal-like solid, but can also appear yellow. Phenanthrene is used to make dyes, plastics, pesticides, explosives, and drugs. It has also been used to make bile acids, cholesterol and steroids.[3]
| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Phenanthrene | |
| Identifiers | |
3D model (JSmol)
|
|
| 1905428 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.437 |
| EC Number |
|
| 28699 | |
| KEGG | |
| MeSH | C031181 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H10 | |
| Molar mass | 178.234 g·mol−1 |
| Appearance | Colorless solid |
| Density | 1.18 g/cm3[1] |
| Melting point | 101 °C (214 °F; 374 K)[1] |
| Boiling point | 332 °C (630 °F; 605 K)[1] |
| 1.6 mg/L[1] | |
| −127.9·10−6 cm3/mol | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 171 °C (340 °F; 444 K)[1] |
| Structure | |
| C2v[2] | |
| 0 D | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phenanthrene occurs naturally and also is a man-made chemical. Commonly, humans are exposed to phenanthrene through inhalation of cigarette smoke, but there are many routes of exposure. Animal studies have shown that phenanthrene is a potential carcinogen.[3] However, according to IARC, it is not identified as a probable, possible or confirmed human carcinogen.[4]
Phenanthrene's three fused rings are angled as in the phenacenes, rather than straight as in the acenes. The compound with a phenanthrene skeleton and nitrogens at the 4 and 5 positions is known as phenanthroline.
Physical Properties
editPhenanthrene is nearly insoluble in water but is soluble in most low-polarity organic solvents such as toluene, carbon tetrachloride, ether, chloroform, acetic acid and benzene.
Phenanthrene is fluorescent under ultraviolet light, exhibiting a large Stoke shift.[5] It can be used in scintillators.
Chemistry
editReactions of phenanthrene typically occur at the 9 and 10 positions, including:
- Organic oxidation to phenanthrenequinone with chromic acid[6]
- Organic reduction to 9,10-dihydrophenanthrene with hydrogen gas and raney nickel[7]
- Electrophilic halogenation to 9-bromophenanthrene with bromine[8]
- Aromatic sulfonation to 2 and 3-phenanthrenesulfonic acids with sulfuric acid[9]
- Ozonolysis to diphenylaldehyde[10]
Canonical forms
editPhenanthrene is more stable than its linear isomer anthracene. A classic and well established explanation is based on Clar's rule. A novel theory invokes so-called stabilizing hydrogen–hydrogen bonds between the C4 and C5 hydrogen atoms.[citation needed]
Synthesis
editThe Bardhan–Sengupta phenanthrene synthesis is a classic way to make phenanthrenes.[11]
This process involves electrophilic aromatic substitution using a tethered cyclohexanol group using diphosphorus pentoxide, which closes the central ring onto an existing aromatic ring. Dehydrogenation using selenium converts the other rings into aromatic ones as well. The aromatization of six-membered rings by selenium is not clearly understood, but it does produce H2Se.
Phenanthrene can also be obtained photochemically from certain diarylethenes (Mallory reaction):
Other synthesis routes include the Haworth reaction and the Wagner-Meerwein-type ring-expansion, as depicted below:
Natural occurrences
editRavatite is a natural mineral consisting of phenanthrene.[12] It is found in small amounts among a few coal burning sites. Ravatite represents a small group of organic minerals.
In plants
editPhenanthrene derivatives occur in plants as phenanthrenoids. They have been reported from flowering plants, mainly in the family Orchidaceae, and a few in the families Dioscoreaceae, Combretaceae and Betulaceae, as well as in the lower plant class Marchantiophyta (liverworts).[13]
See also
editReferences
edit- ^ a b c d e Record of CAS RN 85-01-8 in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ Peter Atkins, J. D. P., Atkins' Physical Chemistry. Oxford: 2010. P. 443.
- ^ a b "Phenanthrene Fact Sheet" (PDF). archive.epa.gov. U.S. Environmental Protection Agency. Retrieved 19 July 2019.
- ^ "Phenanthrene". Sigma-Alrdich.
- ^ "Spectrum [Phenanthrene] | AAT Bioquest". www.aatbio.com. Retrieved 2024-07-30.
- ^ Organic Syntheses, Coll. Vol. 4, p. 757 (1963); Vol. 34, p. 76 (1954).
- ^ Organic Syntheses, Coll. Vol. 4, p. 313 (1963); Vol. 34, p. 31 (1954).
- ^ Organic Syntheses, Coll. Vol. 3, p. 134 (1955); Vol. 28, p. 19 (1948).
- ^ Organic Syntheses, Coll. Vol. 2, p. 482 (1943); Vol. 16, p. 63 (1936).
- ^ Organic Syntheses, Coll. Vol. 5, p. 489 (1973); Vol. 41, p. 41 (1961).
- ^ "Bardhan Sengupta Synthesis". Comprehensive Organic Name Reactions and Reagents. Vol. 49. 2010. pp. 215–219. doi:10.1002/9780470638859.conrr049. ISBN 9780470638859.
- ^ Ravatite Mineral Data
- ^ Kovács, Adriána; Vasas, Andrea; Hohmann, Judit (2008). "Natural phenanthrenes and their biological activity". Phytochemistry. 69 (5): 1084–1110. Bibcode:2008PChem..69.1084K. doi:10.1016/j.phytochem.2007.12.005. PMID 18243254.
External links
edit- Phenanthrene at scorecard.org
