A benign tumor is a mass of cells (tumor) that does not invade neighboring tissue or metastasize (spread throughout the body). Compared to malignant (cancerous) tumors, benign tumors generally have a slower growth rate. Benign tumors have relatively well differentiated cells. They are often surrounded by an outer surface (fibrous sheath of connective tissue) or stay contained within the epithelium. Common examples of benign tumors include moles and uterine fibroids.
| Benign tumor | |
|---|---|
| Other names | non-cancerous tumor |
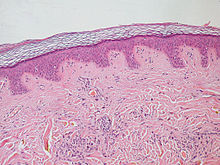 | |
| Normal epidermis and dermis with intradermal nevus, 10x-cropped | |
| Specialty | Oncology, Pathology |
Some forms of benign tumors may be harmful to health. Benign tumor growth causes a mass effect that can compress neighboring tissues. This can lead to nerve damage, blood flow reduction (ischemia), tissue death (necrosis), or organ damage. The health effects of benign tumor growth may be more prominent if the tumor is contained within an enclosed space such as the cranium, respiratory tract, sinus, or bones. For example, unlike most benign tumors elsewhere in the body, benign brain tumors can be life-threatening. Tumors may exhibit behaviors characteristic of their cell type of origin; as an example, endocrine tumors such as thyroid adenomas and adrenocortical adenomas may overproduce certain hormones.
The word "benign" means "favourable, kind, fortunate, salutary, propitious".[1] However, a benign tumour is not benign in the usual sense; the name merely specifies that it is not "malignant", i.e. cancerous. While benign tumours usually do not pose a serious health risk, they can be harmful or fatal.[2] Many types of benign tumors have the potential to become cancerous (malignant) through a process known as tumor progression. For this reason and other possible harms, some benign tumors are removed by surgery. When removed, benign tumors usually do not return. Exceptions to this rule may indicate malignant transformation.
Signs and symptoms
editBenign tumors are very diverse; they may be asymptomatic or may cause specific symptoms, depending on their anatomic location and tissue type. They grow outward, producing large, rounded masses which can cause what is known as a "mass effect". This growth can cause compression of local tissues or organs, leading to many effects, such as blockage of ducts, reduced blood flow (ischaemia), tissue death (necrosis) and nerve pain or damage.[3] Some tumors also produce hormones that can lead to life-threatening situations. Insulinomas can produce large amounts of insulin, causing hypoglycemia.[4][5] Pituitary adenomas can cause elevated levels of hormones such as growth hormone and insulin-like growth factor-1, which cause acromegaly; prolactin; ACTH and cortisol, which cause Cushing's disease; TSH, which causes hyperthyroidism; and FSH and LH.[6] Bowel intussusception can occur with various benign colonic tumors.[7] Cosmetic effects can be caused by tumors, especially those of the skin, possibly causing psychological or social discomfort for the person with the tumor.[8] Vascular tissue tumors can bleed, in some cases leading to anemia.[9]
Causes
editPTEN hamartoma syndrome
editPTEN hamartoma syndrome encompasses hamartomatous disorders characterized by genetic mutations in the PTEN tumor suppressor gene,[10] including Cowden syndrome, Bannayan–Riley–Ruvalcaba syndrome, Proteus syndrome and Proteus-like syndrome. Absent or dysfunctional PTEN protein allows cells to over-proliferate, causing hamartomas.[11] Cowden syndrome is an autosomal dominant genetic disorder characterized by multiple benign hamartomas (trichilemmomas and mucocutaneous papillomatous papules) as well as a predisposition for cancers of multiple organs including the breast and thyroid.[12][13] Bannayan–Riley–Ruvalcaba syndrome is a congenital disorder characterized by hamartomatous intestinal polyposis, macrocephaly, lipomatosis, hemangiomatosis and glans penis macules.[11][14] Proteus syndrome is characterized by nevi, asymmetric overgrowth of various body parts, adipose tissue dysregulation, cystadenomas, adenomas, vascular malformation.[15][16]
Familial adenomatous polyposis
editFamilial adenomatous polyposis (FAP) is a familial cancer syndrome caused by mutations in the APC gene. In FAP, adenomatous polyps are present in the colon. The polyps progress into colon cancer unless removed.[17] The APC gene is a tumor suppressor. Its protein product is involved in many cellular processes. Inactivation of the APC gene leads to the buildup of a protein called β-catenin. This protein activates two transcription factors: T-cell factor (TCF) and lymphoid enhancer factor (LEF). These factors cause the upregulation of many genes involved in cell proliferation, differentiation, migration and apoptosis (programmed cell death), causing the growth of benign tumors.[18]
Tuberous sclerosis complex
editTuberous sclerosis complex (TSC) is an autosomal dominant genetic disorder caused by mutations in the genes TSC1 and TSC2. TSC1 produces the protein hamartin. TSC2 produces the protein tuberin. This disorder presents with many benign hamartomatous tumors including angiofibromas, renal angiomyolipomas, and pulmonary lymphangiomyomatosis. Tuberin and hamartin inhibit the mTOR protein in normal cellular physiology. Inactivation of the TSC tumor suppressors causes an increase in mTOR activity. This leads to the activation of genes and the production of proteins that increase cell growth.[19][20][21]
Von Hippel–Lindau disease
editVon Hippel–Lindau disease is a dominantly inherited cancer syndrome that significantly increases the risk of various tumors. This includes benign hemangioblastomas and malignant pheochromocytomas, renal cell carcinomas, pancreatic endocrine tumors, and endolymphatic sac tumors. It is caused by genetic mutations in the Von Hippel–Lindau tumor suppressor gene. The VHL protein (pVHL) is involved in cellular signaling in oxygen starved (hypoxic) cells. One role of pVHL is to cause the cellular degradation of another protein, HIF1α. Dysfunctional pVHL leads to accumulation of HIF1α. This activates several genes responsible for the production of substances involved in cell growth and blood vessel production: VEGF, PDGFβ, TGFα and erythropoietin.[22]
Bone tumors
editBenign tumors of bone can be similar macroscopically and require a combination of a clinical history with cytogenetic, molecular, and radiologic tests for diagnosis.[23] Three common forms of benign bone tumors with are giant cell tumor of bone, osteochondroma, and enchondroma; other forms of benign bone tumors exist but may be less prevalent.
Giant cell tumors
editGiant cell tumors of bone frequently occur in long bone epiphyses of the appendicular skeleton or the sacrum of the axial skeleton. Local growth can cause destruction of neighboring cortical bone and soft tissue, leading to pain and limiting range of motion. The characteristic radiologic finding of giant cell tumors of bone is a lytic lesion that does not have marginal sclerosis of bone. On histology, giant cells of fused osteoclasts are seen as a response to neoplastic mononucleated cells. Notably, giant cells are not unique among benign bone tumors to giant cell tumors of bone. Molecular characteristics of the neoplastic cells causing giant cell tumors of bone indicate an origin of pluripotent mesenchymal stem cells that adopt preosteoblastic markers. Cytogenetic causes of giant cell tumors of bone involve telomeres. Treatment involves surgical curettage with adjuvant bisphosphonates.
Osteochondroma
editOsteochondromas form cartilage-capped projections of bone. Structures such as the marrow cavity and cortical bone of the osteochondroma are contiguous to those of the originating bone. Sites of origin often involve metaphyses of long bones. While many osteochondromas occur spontaneously, there are cases in which several osteochondromas can occur in the same individual; these may be linked to a genetic condition known as hereditary multiple osteochondromas. Osteochondroma appears on X-ray as a projecting mass that often points away from joints.[23] These tumors stop growing with the closure of the parental bone's growth plates. Failure to stop growth can be indicative of transformation to malignant chondrosarcoma. Treatment is not indicated unless symptomatic. In that case, surgical excision is often curative.
Enchondroma
editEnchondromas are benign tumors of hyaline cartilage. Within a bone, enchondromas are often found in metaphyses. They can be found in many types of bone, including small bones, long bones, and the axial skeleton. X-ray of enchondromas shows well-defined borders and a stippled appearance.[23] Presentation of multiple enchondromas is consistent with multiple enchondromatosis (Ollier Disease). Treatment of enchondromas involves surgical curettage and grafting.
Benign soft tissue tumors
editLipomas
editLipomas are benign, subcutaneous tumors of fat cells (adipocytes). They are usually painless, slow-growing, and mobile masses that can occur anywhere in the body where there are fat cells, but are typically found on the trunk and upper extremities.[24]
[25] Although lipomas can develop at any age, they more commonly appear between the ages of 40 and 60.[24] Lipomas affect about 1% of the population, with no documented sex bias, and about 1 in every 1000 people will have a lipoma within their lifetime.[25][26] The cause of lipomas is not well defined. Genetic or inherited causes of lipomas play a role in around 2-3% of patients.[25] In individuals with inherited familial syndromes such as Proteus syndrome or Familial multiple lipomatosis, it is common to see multiple lipomas across the body.[25] These syndromes are also associated with specific symptoms and sub-populations. Mutations in chromosome 12 have been identified in around 65% of lipoma cases.[25] Lipomas have also been shown to be increased in those with obesity, hyperlipidemia, and diabetes mellitus.[25]
Lipomas are usually diagnosed clinically, although imaging (ultrasound, computed tomography, or magnetic resonance imaging) may be utilized to assist with the diagnosis of lipomas in atypical locations.[24] The main treatment for lipomas is surgical excision, after which the tumor is examined with histopathology to confirm the diagnosis.[24] The prognosis for benign lipomas is excellent and recurrence after excision is rare, but may occur if the removal was incomplete.[25]
Mechanism
editBenign vs malignant
editOne of the most important factors in classifying a tumor as benign or malignant is its invasive potential. If a tumor lacks the ability to invade adjacent tissues or spread to distant sites by metastasizing then it is benign, whereas invasive or metastatic tumors are malignant.[3] For this reason, benign tumors are not classed as cancer.[27] Benign tumors will grow in a contained area usually encapsulated in a fibrous connective tissue capsule. The growth rates of benign and malignant tumors also differ; benign tumors generally grow more slowly than malignant tumors. Although benign tumors pose a lower health risk than malignant tumors, they both can be life-threatening in certain situations. There are many general characteristics which apply to either benign or malignant tumors, but sometimes one type may show characteristics of the other. For example, benign tumors are mostly well differentiated and malignant tumors are often undifferentiated. However, undifferentiated benign tumors and differentiated malignant tumors can occur.[28][29] Although benign tumors generally grow slowly, cases of fast-growing benign tumors have also been documented.[30] Some malignant tumors are mostly non-metastatic such as in the case of basal-cell carcinoma.[31] CT and chest radiography can be a useful diagnostic exam in visualizing a benign tumor and differentiating it from a malignant tumor. The smaller the tumor on a radiograph the more likely it is to be benign as 80% of lung nodules less than 2 cm in diameter are benign. Most benign nodules are smoothed radiopaque densities with clear margins but these are not exclusive signs of benign tumors.[32]
Multistage carcinogenesis
editTumors are formed by carcinogenesis, a process in which cellular alterations lead to the formation of cancer. Multistage carcinogenesis involves the sequential genetic or epigenetic changes to a cell's DNA, where each step produces a more advanced tumor. It is often broken down into three stages; initiation, promotion and progression, and several mutations may occur at each stage. Initiation is where the first genetic mutation occurs in a cell. Promotion is the clonal expansion (repeated division) of this transformed cell into a visible tumor that is usually benign. Following promotion, progression may take place where more genetic mutations are acquired in a sub-population of tumor cells. Progression changes the benign tumor into a malignant tumor.[33][34] A prominent and well studied example of this phenomenon is the tubular adenoma, a common type of colon polyp which is an important precursor to colon cancer. The cells in tubular adenomas, like most tumors that frequently progress to cancer, show certain abnormalities of cell maturation and appearance collectively known as dysplasia. These cellular abnormalities are not seen in benign tumors that rarely or never turn cancerous, but are seen in other pre-cancerous tissue abnormalities which do not form discrete masses, such as pre-cancerous lesions of the uterine cervix.
Diagnosis
editClassification
editBenign neoplasms are typically, but not always, composed of cells which bear a strong resemblance to a normal cell type in their organ of origin. These tumors are named for the cell or tissue type from which they originate. The suffix "-oma" (but not -carcinoma, -sarcoma, or -blastoma, which are generally cancers) is applied to indicate a benign tumor. For example, a lipoma is a common benign tumor of fat cells (lipocytes), and a chondroma is a benign tumor of cartilage-forming cells (chondrocytes). Adenomas are benign tumors of gland-forming cells, and are usually specified further by their cell or organ of origin, as in hepatic adenoma (a benign tumor of hepatocytes, or liver cells). Teratomas contain many cell types such as skin, nerve, brain and thyroid, among others, because they are derived from germ cells.[36] Hamartomas are a group of benign tumors that have relatively normal cellular differentiation but exhibit disorganized tissue organization.[19]
Exceptions to the nomenclature rules exist for historical reasons; malignant examples include melanoma (a cancer of pigmented skin cells, or melanocytes) and seminoma (a cancer of male reproductive cells).[37]
Benign tumors do not encompass all benign growths. Skin tags, vocal chord polyps, and hyperplastic polyps of the colon are often referred to as benign, but they are overgrowths of normal tissue rather than neoplasms.[36]
Treatment
editBenign tumors typically need no treatment unless if they cause problems such as seizures, discomfort or cosmetic concerns. Surgery is usually the most effective approach and is used to treat most benign tumors. In some cases, other treatments may be used. Adenomas of the rectum may be treated with sclerotherapy, in which chemicals are used to shrink blood vessels in order to cut off the blood supply.[38] Most benign tumors do not respond to chemotherapy or radiation therapy, although there are exceptions; benign intercranial tumors are sometimes treated with radiation therapy and chemotherapy under certain circumstances.[39][40] Radiation can also be used to treat hemangiomas in the rectum.[38] Benign skin tumors are usually surgically resected but other treatments such as cryotherapy, curettage, electrodesiccation, laser therapy, dermabrasion, chemical peels and topical medication are used.[41][42]
References
edit- ^ "Benign". Oxford English Dictionary (Online ed.). Oxford University Press. (Subscription or participating institution membership required.)
- ^ Rao AK (February 2021). "Overview of Heart Tumors - Treatment of noncancerous (benign) heart tumors". MSD Manual Consumer Version.
Children with this type of [inoperable, benign] tumor usually die of an abnormal heart rhythm at an early age.
- ^ a b Wilson KA, Waugh A, Chambers G, Grant A, Ross J (2006). Ross and Wilson anatomy and physiology in health and illness. Edinburgh: Churchill Livingstone. pp. 53–54. ISBN 0-443-10101-9.
- ^ Marks V, Teale JD (June 1991). "Tumours producing hypoglycaemia". Diabetes/Metabolism Reviews. 7 (2): 79–91. doi:10.1002/dmr.5610070202. PMID 1665409. S2CID 19272145.
- ^ Grant CS (October 2005). "Insulinoma". Best Practice & Research. Clinical Gastroenterology. 19 (5): 783–798. doi:10.1016/j.bpg.2005.05.008. PMID 16253900.
- ^ Eng C, DeLellis RA, Lloyd RV, Heitz PU (2004). Pathology and genetics of tumours of endocrine organs. Lyon: IARC Press. ISBN 92-832-2416-7.
- ^ Gill SS, Heuman DM, Mihas AA (October 2001). "Small intestinal neoplasms". Journal of Clinical Gastroenterology. 33 (4): 267–282. doi:10.1097/00004836-200110000-00004. PMID 11588539.
- ^ Tromberg J, Bauer B, Benvenuto-Andrade C, Marghoob AA (2005). "Congenital melanocytic nevi needing treatment". Dermatologic Therapy. 18 (2): 136–150. doi:10.1111/j.1529-8019.2005.05012.x. PMID 15953143. S2CID 20915929.
- ^ Zuber M, Harder F (2001). Benign tumors of the colon and rectum. Munich: Zuckschwerdt: Surgical Treatment: Evidence-Based and Problem-Oriented.
- ^ Pilarski R (June 2019). "PTEN Hamartoma Tumor Syndrome: A Clinical Overview". Cancers. 11 (6): 844. doi:10.3390/cancers11060844. PMC 6627214. PMID 31216739.
- ^ a b Hobert JA, Eng C (October 2009). "PTEN hamartoma tumor syndrome: an overview". Genetics in Medicine. 11 (10): 687–694. doi:10.1097/GIM.0b013e3181ac9aea. PMID 19668082.
- ^ Pilarski R, Eng C (May 2004). "Will the real Cowden syndrome please stand up (again)? Expanding mutational and clinical spectra of the PTEN hamartoma tumour syndrome". Journal of Medical Genetics. 41 (5): 323–326. doi:10.1136/jmg.2004.018036. PMC 1735782. PMID 15121767.
- ^ Eng C (November 2000). "Will the real Cowden syndrome please stand up: revised diagnostic criteria". Journal of Medical Genetics. 37 (11): 828–830. doi:10.1136/jmg.37.11.828. PMC 1734465. PMID 11073535.
- ^ Eng C (September 2003). "PTEN: one gene, many syndromes". Human Mutation. 22 (3): 183–198. doi:10.1002/humu.10257. PMID 12938083. S2CID 13417857.
- ^ Blumenthal GM, Dennis PA (November 2008). "PTEN hamartoma tumor syndromes". European Journal of Human Genetics. 16 (11): 1289–1300. doi:10.1038/ejhg.2008.162. PMC 6939673. PMID 18781191.
- ^ Cohen MM (August 2005). "Proteus syndrome: an update". American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 137C (1): 38–52. doi:10.1002/ajmg.c.30063. PMID 16010681. S2CID 31873101.
- ^ Galiatsatos P, Foulkes WD (February 2006). "Familial adenomatous polyposis". The American Journal of Gastroenterology. 101 (2): 385–398. doi:10.1111/j.1572-0241.2006.00375.x. PMID 16454848. S2CID 8516051.
- ^ Aoki K, Taketo MM (October 2007). "Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene". Journal of Cell Science. 120 (Pt 19): 3327–3335. doi:10.1242/jcs.03485. PMID 17881494. S2CID 8743.
- ^ a b Inoki K, Corradetti MN, Guan KL (January 2005). "Dysregulation of the TSC-mTOR pathway in human disease". Nature Genetics. 37 (1): 19–24. doi:10.1038/ng1494. PMID 15624019. S2CID 205344131.
- ^ Crino PB, Nathanson KL, Henske EP (September 2006). "The tuberous sclerosis complex". The New England Journal of Medicine. 355 (13): 1345–1356. doi:10.1056/NEJMra055323. PMID 17005952. S2CID 3579356.
- ^ Kwiatkowski DJ (January 2003). "Tuberous sclerosis: from tubers to mTOR". Annals of Human Genetics. 67 (Pt 1): 87–96. doi:10.1046/j.1469-1809.2003.00012.x. PMID 12556239. S2CID 41992893.
- ^ Maher ER (December 2004). "Von Hippel–Lindau disease". Current Molecular Medicine. 4 (8): 833–842. doi:10.2174/1566524043359827. PMID 15579030.
- ^ a b c Garcia RA, Inwards CY, Unni KK (February 2011). "Benign bone tumors--recent developments". Seminars in Diagnostic Pathology. Orthopaedic Pathology. 28 (1): 73–85. doi:10.1053/j.semdp.2011.02.013. PMID 21675379.
- ^ a b c d Lichon, Sabrina; Khachemoune, Amor (September 2018). "Clinical presentation, diagnostic approach, and treatment of hand lipomas: a review". Acta Dermatovenerologica Alpina, Pannonica, et Adriatica. 27 (3): 137–139. ISSN 1581-2979. PMID 30244263.
- ^ a b c d e f g Kolb, Logan; Yarrarapu, Siva Naga S.; Ameer, Muhammad Atif; Rosario-Collazo, Juan A. (2022), "Lipoma", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 29939683, retrieved 2022-09-19
- ^ Charifa, Ahmad; Azmat, Chaudhary Ehtsham; Badri, Talel (2022), "Lipoma Pathology", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 29493968, retrieved 2022-09-19
- ^ Nunn LS, Silverstein A, Silverstein VB (2006). Cancer. Brookfield, Conn: Twenty-First Century Books. pp. 11–12. ISBN 0-7613-2833-5.
- ^ Skorić T, Korsić M, Zarković K, Plavsić V, Besenski N, Breskovac L, et al. (June 1999). "Clinical and morphological features of undifferentiated monomorphous GH/TSH-secreting pituitary adenoma". European Journal of Endocrinology. 140 (6): 528–537. doi:10.1530/eje.0.1400528. PMID 10366409.
- ^ Song HJ, Xue YL, Qiu ZL, Luo QY (2012). "Uncommon metastases from differentiated thyroid carcinoma". Hellenic Journal of Nuclear Medicine. 15 (3): 233–240. PMID 23106056.
- ^ Sagel SS, Ablow RC (November 1968). "Hamartoma: on occasion a rapidly growing tumor of the lung". Radiology. 91 (5): 971–972. doi:10.1148/91.5.971. PMID 5681331.
- ^ Strayer DL, Rubin R, Rubin E (2008). Rubin's pathology: clinicopathologic foundations of medicine. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins. pp. 138–139. ISBN 978-1605479682.
- ^ Erasmus JJ, Connolly JE, McAdams HP, Roggli VL (2000). "Solitary pulmonary nodules: Part I. Morphologic evaluation for differentiation of benign and malignant lesions". Radiographics. 20 (1): 43–58. doi:10.1148/radiographics.20.1.g00ja0343. PMID 10682770.
- ^ Clark WH (October 1991). "Tumour progression and the nature of cancer". British Journal of Cancer. 64 (4): 631–644. doi:10.1038/bjc.1991.375. PMC 1977704. PMID 1911211.
- ^ Barrett JC (April 1993). "Mechanisms of multistep carcinogenesis and carcinogen risk assessment". Environmental Health Perspectives. 100: 9–20. doi:10.1289/ehp.931009. PMC 1519586. PMID 8354184.
- ^ Wujcik D, Yarbro CH, Gobel BH (2011). Cancer nursing: principles and practice. Boston: Jones and Bartlett Publishers. ISBN 978-0-7637-6357-2.
- ^ a b Strayer DL, Rubin R, Rubin E (2008). Rubin's pathology: clinicopathologic foundations of medicine. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins. pp. 138–139. ISBN 978-0-7817-9516-6.
- ^ Cotran R, Kumar V, Collins T (1999). Robbins Pathologic Basis of Disease (6th ed.). W.B. Saunders. ISBN 0-7216-7335-X.
- ^ a b Zuber M, Harder F (2001). Benign tumors of the colon and rectum. Munich: Zuckschwerdt: Surgical Treatment: Evidence-Based and Problem-Oriented.
- ^ Brada M (February 2013). "Radiotherapy for benign brain tumours coming of age; example of vestibular schwannoma". Radiotherapy and Oncology. 106 (2): 157–160. doi:10.1016/j.radonc.2013.01.009. PMID 23462704.
- ^ Sioka C, Kyritsis AP (March 2009). "Chemotherapy, hormonal therapy, and immunotherapy for recurrent meningiomas". Journal of Neuro-Oncology. 92 (1): 1–6. doi:10.1007/s11060-008-9734-y. PMID 19023520. S2CID 28106960.
- ^ Luba MC, Bangs SA, Mohler AM, Stulberg DL (February 2003). "Common benign skin tumors". American Family Physician. 67 (4): 729–738. PMID 12613727.
- ^ Marghoob AA, Borrego JP, Halpern AC (December 2007). "Congenital melanocytic nevi: treatment modalities and management options". Seminars in Cutaneous Medicine and Surgery. 26 (4): 231–240. doi:10.1016/j.sder.2008.03.007. PMID 18395671.