Boron trioxide or diboron trioxide is the oxide of boron with the formula B2O3. It is a colorless transparent solid, almost always glassy (amorphous), which can be crystallized only with great difficulty. It is also called boric oxide[6] or boria.[7] It has many important industrial applications, chiefly in ceramics as a flux for glazes and enamels and in the production of glasses.
![Crystal structure of B2O3 [1]](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d9/B2O3powder.JPG/220px-B2O3powder.JPG)
| |
| |
| Names | |
|---|---|
| IUPAC name
Diboron trioxide
| |
| Other names
boron oxide, diboron trioxide, boron sesquioxide, boric oxide, boria
Boric anhydride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.751 |
| EC Number |
|
| 11108 | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| B2O3 | |
| Molar mass | 69.6182 g/mol |
| Appearance | white, glassy solid |
| Density | 2.460 g/cm3, liquid; 2.55 g/cm3, trigonal; |
| Melting point | 450 °C (842 °F; 723 K) (trigonal) 510 °C (tetrahedral) |
| Boiling point | 1,860 °C (3,380 °F; 2,130 K) ,[2] sublimes at 1500 °C[3] |
| 1.1 g/100mL (10 °C) 3.3 g/100mL (20 °C) 15.7 g/100mL (100 °C) | |
| Solubility | partially soluble in methanol |
| Acidity (pKa) | ~ 4 |
| −39.0·10−6 cm3/mol | |
| Thermochemistry | |
Heat capacity (C)
|
66.9 J/(mol⋅K) |
Std molar
entropy (S⦵298) |
80.8 J/(mol⋅K) |
Std enthalpy of
formation (ΔfH⦵298) |
−1254 kJ/mol |
Gibbs free energy (ΔfG⦵)
|
−832 kJ/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant[4] |
| GHS labelling: | |

| |
| Danger | |
| H360FD | |
| P201, P202, P281, P308+P313, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | noncombustible |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
3163 mg/kg (oral, mouse)[5] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 15 mg/m3[4] |
REL (Recommended)
|
TWA 10 mg/m3[4] |
IDLH (Immediate danger)
|
2000 mg/m3[4] |
| Supplementary data page | |
| Boron trioxide (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Structure
editBoron trioxide has three known forms, one amorphous and two crystalline.
Amorphous form
editThe amorphous form (g-B2O3) is by far the most common. It is thought to be composed of boroxol rings which are six-membered rings composed of alternating 3-coordinate boron and 2-coordinate oxygen.
Because of the difficulty of building disordered models at the correct density with many boroxol rings, this view was initially controversial, but such models have recently been constructed and exhibit properties in excellent agreement with experiment.[8][9] It is now recognized, from experimental and theoretical studies,[10][11][12][13][14] that the fraction of boron atoms belonging to boroxol rings in glassy B2O3 is somewhere between 0.73 and 0.83, with 0.75 = 3/4 corresponding to a 1:1 ratio between ring and non-ring units. The number of boroxol rings decays in the liquid state with increasing temperature.[15]
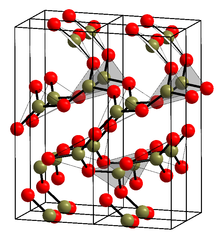
Crystalline α form
editThe crystalline form (α-B2O3) is exclusively composed of BO3 triangles. It crystal structure was initially believed to be the enantiomorphic space groups P31(#144) and P32(#145), like γ-glycine;[16][17] but was later revised to the enantiomorphic space groups P3121(#152) and P3221(#154) in the trigonal crystal system, like α-quartz[18]
Crystallization of α-B2O3 from the molten state at ambient pressure is strongly kinetically disfavored (compare liquid and crystal densities). It can be obtained with prologued annealing of the amorphous solid ~200 °C under at least 10 kbar of pressure.[19][1]
Crystalline β form
editThe trigonal network undergoes a coesite-like transformation to monoclinic β-B2O3 at several gigapascals (9.5 GPa).[20]
Preparation
editBoron trioxide is produced by treating borax with sulfuric acid in a fusion furnace. At temperatures above 750 °C, the molten boron oxide layer separates out from sodium sulfate. It is then decanted, cooled and obtained in 96–97% purity.[3]
Another method is heating boric acid above ~300 °C. Boric acid will initially decompose into steam, (H2O(g)) and metaboric acid (HBO2) at around 170 °C, and further heating above 300 °C will produce more steam and diboron trioxide. The reactions are:
- H3BO3 → HBO2 + H2O
- 2 HBO2 → B2O3 + H2O
Boric acid goes to anhydrous microcrystalline B2O3 in a heated fluidized bed.[21] Carefully controlled heating rate avoids gumming as water evolves.
Boron oxide will also form when diborane (B2H6) reacts with oxygen in the air or trace amounts of moisture:
- 2B2H6(g) + 3O2(g) → 2B2O3(s) + 6H2(g)
- B2H6(g) + 3H2O(g) → B2O3(s) + 6H2(g)[22]
Reactions
editMolten boron oxide attacks silicates. Containers can be passivated internally with a graphitized carbon layer obtained by thermal decomposition of acetylene.[23]
Applications
edit- Major component of borosilicate glass
- Fluxing agent for glass and enamels[citation needed]
- An additive used in glass fibres (optical fibres)
- The inert capping layer in the Liquid Encapsulation Czochralski process for the production of gallium arsenide single crystal
- As an acid catalyst in organic synthesis
- As a starting material for the production of other boron compounds, such as boron carbide
See also
editReferences
edit- ^ a b Gurr, G. E.; Montgomery, P. W.; Knutson, C. D.; Gorres, B. T. (1970). "The Crystal Structure of Trigonal Diboron Trioxide". Acta Crystallographica B. 26 (7): 906–915. doi:10.1107/S0567740870003369.
- ^ High temperature corrosion and materials chemistry: proceedings of the Per Kofstad Memorial Symposium. Proceedings of the Electrochemical Society. The Electrochemical Society. 2000. p. 496. ISBN 978-1-56677-261-7.
- ^ a b Patnaik, P. (2003). Handbook of Inorganic Chemical Compounds. McGraw-Hill. p. 119. ISBN 978-0-07-049439-8. Retrieved 2009-06-06.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0060". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Boron oxide". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ L. McCulloch (1937): "A Crystalline Boric Oxide". Journal of the American Chemical Society, volume 59, issue 12, pages 2650–2652. doi:10.1021/ja01291a05
- ^ I.Vishnevetsky and M.Epstein (2015): "Solar carbothermic reduction of alumina, magnesia and boria under vacuum". Solar Energy, volume 111, pages 236-251 doi:10.1016/j.solener.2014.10.039
- ^ Ferlat, G.; Charpentier, T.; Seitsonen, A. P.; Takada, A.; Lazzeri, M.; Cormier, L.; Calas, G.; Mauri. F. (2008). "Boroxol Rings in Liquid and Vitreous B2O3 from First Principles". Phys. Rev. Lett. 101 (6): 065504. Bibcode:2008PhRvL.101f5504F. doi:10.1103/PhysRevLett.101.065504. PMID 18764473.
- ^ Ferlat, G.; Seitsonen, A. P.; Lazzeri, M.; Mauri, F. (2012). "Hidden polymorphs drive vitrification in B2O3". Nature Materials Letters. 11 (11): 925–929. arXiv:1209.3482. Bibcode:2012NatMa..11..925F. doi:10.1038/NMAT3416. PMID 22941329. S2CID 11567458.
- ^ Hung, I.; et al. (2009). "Determination of the bond-angle distribution in vitreous B2O3 by rotation (DOR) NMR spectroscopy". Journal of Solid State Chemistry. 182 (9): 2402–2408. Bibcode:2009JSSCh.182.2402H. doi:10.1016/j.jssc.2009.06.025.
- ^ Soper, A. K. (2011). "Boroxol rings from diffraction data on vitreous boron trioxide". J. Phys.: Condens. Matter. 23 (36): 365402. Bibcode:2011JPCM...23.5402S. doi:10.1088/0953-8984/23/36/365402. PMID 21865633. S2CID 5291179.
- ^ Joo, C.; et al. (2000). "The ring structure of boron trioxide glass". Journal of Non-Crystalline Solids. 261 (1–3): 282–286. Bibcode:2000JNCS..261..282J. doi:10.1016/s0022-3093(99)00609-2.
- ^ Zwanziger, J. W. (2005). "The NMR response of boroxol rings: a density functional theory study". Solid State Nuclear Magnetic Resonance. 27 (1–2): 5–9. doi:10.1016/j.ssnmr.2004.08.004. PMID 15589722.
- ^ Micoulaut, M. (1997). "The structure of vitreous B2O3 obtained from a thermostatistical model of agglomeration". Journal of Molecular Liquids. 71 (2–3): 107–114. doi:10.1016/s0167-7322(97)00003-2.
- ^ Alderman, O. L. G. Ferlat, G. Baroni, A. Salanne, M. Micoulaut, M. Benmore, C. J. Lin, A. Tamalonis, A. Weber, J. K. R. (2015). "Liquid B2O3 up to 1700K: X-ray diffraction and boroxol ring dissolution" (PDF). Journal of Physics: Condensed Matter. 27 (45): 455104. Bibcode:2015JPCM...27S5104A. doi:10.1088/0953-8984/27/45/455104. PMID 26499978. S2CID 21783488.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gurr, G. E.; Montgomery, P. W.; Knutson, C. D.; Gorres, B. T. (1970). "The crystal structure of trigonal diboron trioxide". Acta Crystallographica B. 26 (7): 906–915. doi:10.1107/S0567740870003369.
- ^ Strong, S. L.; Wells, A. F.; Kaplow, R. (1971). "On the crystal structure of B2O3". Acta Crystallographica B. 27 (8): 1662–1663. doi:10.1107/S0567740871004515.
- ^ Effenberger, H.; Lengauer, C. L.; Parthé, E. (2001). "Trigonal B2O3 with Higher Space-Group Symmetry: Results of a Reevaluation". Monatshefte für Chemie. 132 (12): 1515–1517. doi:10.1007/s007060170008. S2CID 97795834.
- ^ Aziz, M. J.; Nygren, E.; Hays, J. F.; Turnbull, D. (1985). "Crystal Growth Kinetics of Boron Oxide Under Pressure". Journal of Applied Physics. 57 (6): 2233. Bibcode:1985JAP....57.2233A. doi:10.1063/1.334368.
- ^ Brazhkin, V. V.; Katayama, Y.; Inamura, Y.; Kondrin, M. V.; Lyapin, A. G.; Popova, S. V.; Voloshin, R. N. (2003). "Structural transformations in liquid, crystalline and glassy B2O3 under high pressure". JETP Letters. 78 (6): 393–397. Bibcode:2003JETPL..78..393B. doi:10.1134/1.1630134. S2CID 189764568.
- ^ Kocakuşak, S.; Akçay, K.; Ayok, T.; Koöroğlu, H. J.; Koral, M.; Savaşçi, Ö. T.; Tolun, R. (1996). "Production of anhydrous, crystalline boron oxide in fluidized bed reactor". Chemical Engineering and Processing. 35 (4): 311–317. doi:10.1016/0255-2701(95)04142-7.
- ^ AirProducts (2011). "Diborane Storage & Delivery" (PDF). Archived from the original (PDF) on 2015-02-04. Retrieved 2013-08-21.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Morelock, C. R. (1961). "Research Laboratory Report #61-RL-2672M". General Electric.
{{cite journal}}: Cite journal requires|journal=(help)
