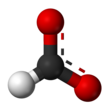
Formate (IUPAC name: methanoate) is the conjugate base of formic acid. Formate is an anion (HCO−2) or its derivatives such as ester of formic acid. The salts and esters are generally colorless.[1]

| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Formate | |||
| Systematic IUPAC name
Methanoate | |||
| Other names
Formylate
Methylate Isocarbonite Carbonite(1-) Hydrogencarboxylate Metacarbonoate Oxocarbinate Oxomethyl oxide ion Oxomethoxide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| MeSH | Formates | ||
PubChem CID
|
|||
| UNII | |||
| |||
| Properties | |||
| HCOO− or HCO− 2 | |||
| Molar mass | 45.017 g mol−1 | ||
| Conjugate acid | Formic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Fundamentals
editWhen dissolved in water, formic acid converts to formate:
- HCO2H → HCO−2 + H+
Formate is a planar anion. The two oxygen atoms are equivalent and bear a partial negative charge. The remaining C-H bond is not acidic.
Biochemistry
editFormate is a common C-1 source in living systems. It is formed from many precursors including choline, serine, and sarcosine. It provides a C-1 source in the biosynthesis of some nucleic acids. Formate (or formic acid) is invoked as a leaving group in the demethylation of some sterols.[2] These conversions are catalyzed by aromatase enzymes using O2 as the oxidant. Specific conversions include testosterone to estradiol and androstenedione to estrone.[3]
Formate is reversibly oxidized by the enzyme formate dehydrogenase from Desulfovibrio gigas:[4]
- HCO−2 → CO2 + H+ + 2 e−
Formate esters
editFormate esters have the formula HCOOR (alternative way of writing formula ROC(O)H or RO2CH). Many form spontaneously when alcohols dissolve in formic acid.
The most important formate ester is methyl formate, which is produced as an intermediate en route to formic acid. Methanol and carbon monoxide react in the presence of a strong base, such as sodium methoxide:[1]
- CH3OH + CO → HCOOCH3
Hydrolysis of methyl formate gives formic acid and regenerates methanol:
- HCOOCH3 → HCOOH + CH3OH
Formic acid is used for many applications in industry.
Formate esters often are fragrant or have distinctive odors. Compared to the more common acetate esters, formate esters are less commonly used commercially because they are less stable.[5] Ethyl formate is found in some confectionaries.[1]
Formate salts
editFormate salts have the formula M(O2CH)(H2O)x. Such salts are prone to decarboxylation. For example, hydrated nickel formate decarboxylates at about 200 °C with reduction of the Ni2+ to finely powdered nickel metal:
- Ni(HCO2)2(H2O)2 → Ni + 2 CO2 + 2 H2O + H2
Such fine powders are useful as hydrogenation catalysts.[1]
Examples
edit- ethyl formate, CH3CH2(HCOO)
- sodium formate, Na(HCOO)
- potassium formate, K(HCOO)
- caesium formate, Cs(HCOO); see Caesium: Petroleum exploration
- methyl formate, CH3(HCOO)
- methyl chloroformate, CH3OCOCl
- triethyl orthoformate
- trimethyl orthoformate, C4H10O3
- phenyl formate HCOOC6H5
- amyl formate
References
edit- ^ a b c d Reutemann, Werner; Kieczka, Heinz (2000), "Formic Acid", Ullmann's Encyclopedia of Industrial Chemistry, doi:10.1002/14356007.a12_013, ISBN 3-527-30673-0
- ^ Pietzke, Matthias; Meiser, Johannes; Vazquez, Alexei (2020). "Formate Metabolism in Health and Disease". Molecular Metabolism. 33: 23–37. doi:10.1016/j.molmet.2019.05.012. PMC 7056922. PMID 31402327.
- ^ Lephart, E. D. (1996). "A Review of Brain Aromatase Cytochrome P450". Brain Res. Rev. 22 (1): 1–26. doi:10.1016/0165-0173(96)00002-1. PMID 8871783. S2CID 11987113.
- ^ Reda, Torsten; Plugge, Caroline M.; Abram, Nerilie J.; Hirst, Judy (2008). "Reversible interconversion of carbon dioxide and formate by an electroactive enzyme". Proceedings of the National Academy of Sciences. 105 (31): 10654–10658. doi:10.1073/pnas.0801290105. PMC 2491486. PMID 18667702.
- ^ Panten, Johannes; Surburg, Horst (2015), "Flavors and Fragrances, 2. Aliphatic Compounds", Ullmann's Encyclopedia of Industrial Chemistry, pp. 1–55, doi:10.1002/14356007.t11_t01, ISBN 978-3-527-30673-2