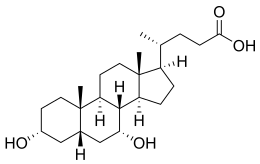
Chenodeoxycholic acid (CDCA; also known as chenodesoxycholic acid, chenocholic acid and 3α,7α-dihydroxy-5β-cholan-24-oic acid) is a bile acid. Salts of this carboxylic acid are called chenodeoxycholates. Chenodeoxycholic acid is one of the main bile acids.[1][2][3] It was first isolated from the bile of the domestic goose, which gives it the "cheno" portion of its name (Greek: χήν = goose).[4]
| |

| |
| Names | |
|---|---|
| IUPAC name
3α,7α-Dihydroxy-5β-cholan-24-oic acid
| |
| Systematic IUPAC name
(4R)-4-[(1R,3aS,3bR,4R,5aS,7R,9aS,9bS,11aR)-4,7-Dihydroxy-9a,11a-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-1-yl]pentanoic acid | |
| Other names
Chenodiol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.006.803 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C24H40O4 | |
| Molar mass | 392.57 g/mol |
| Melting point | 165 to 167 °C (329 to 333 °F; 438 to 440 K) |
| Pharmacology | |
| A05AA01 (WHO) | |
| License data | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Structure
editChenodeoxycholic acid and cholic acid are the two primary bile acids in humans. Chenodeoxycholic acid has two hydroxyl groups and is modified with the addition of another hydroxyl group to produce cholic acid. Some other mammals have muricholic acid or deoxycholic acid rather than chenodeoxycholic acid.[1] It occurs as a white crystalline substance insoluble in water but soluble in alcohol and acetic acid, with melting point at 165–167 °C.[citation needed]
Biosynthesis and function
editChenodeoxycholic acid is synthesized in the liver from cholesterol via several enzymatic steps.[1] Like other bile acids, it can be conjugated with taurine or glycine, forming taurochenodeoxycholate or glycochenodeoxycholate. Conjugation results in a lower pKa. This results in the conjugated bile acids being ionized at the usual pH in the intestine, and staying in the gastrointestinal tract until reaching the ileum to be reabsorbed.[3]
CDCA and other bile acids are surfactants forming micelles with fats, which facilitate lipid digestion. After absorption, they are taken up by the liver and resecreted, so undergoing an enterohepatic circulation. Unabsorbed CDCA can be metabolised by bacteria in the colon to form the secondary bile acid, lithocholic acid or the epimer, ursodeoxycholic acid.[3]
CDCA is the most potent natural bile acid at stimulating the nuclear bile acid receptor, farnesoid X receptor (FXR).[5] The transcription of many genes is activated by FXR, including those encoding FGF19 and small heterodimer partner.[6]
Therapeutic applications
editGallstones
editCDCA has been used as medical therapy to dissolve gallstones.[7][8] Medical therapy with oral bile acids has been used in patients who have small cholesterol stones, and for patients with larger cholesterol gallstones who are unable or reluctant to have surgery. CDCA treatment can cause diarrhea, mild reversible hepatic injury, and a small increase in the plasma cholesterol level.[8]
Cerebrotendineous xanthomatosis
editCDCA can be used in the treatment of cerebrotendineous xanthomatosis.[9]
Other
editCDCA has been used in several other conditions.[10] As diarrhea is frequent when CDCA is used in gallstone dissolution, it has been studied as a possible treatment for constipation and has been shown to accelerate colonic transit and improve bowel function.[11]
The Australian biotechnology company Giaconda has tested a treatment for hepatitis C infection that combines chenodeoxycholic acid with bezafibrate.[12]
See also
editReferences
edit- ^ a b c Russell DW (2003). "The enzymes, regulation, and genetics of bile acid synthesis". Annu. Rev. Biochem. 72: 137–74. doi:10.1146/annurev.biochem.72.121801.161712. PMID 12543708.
- ^ Bhagavan, N.V.; Ha, Chung-Eun (2015). "Gastrointestinal Digestion and Absorption". Essentials of Medical Biochemistry. pp. 137–164. doi:10.1016/B978-0-12-416687-5.00011-7. ISBN 9780124166875.
- ^ a b c Dawson, PA; Karpen, SJ (June 2015). "Intestinal transport and metabolism of bile acids". Journal of Lipid Research. 56 (6): 1085–99. doi:10.1194/jlr.R054114. PMC 4442867. PMID 25210150.
- ^ Carey MC (December 1975). "Editorial: Cheno and urso: what the goose and the bear have in common". N. Engl. J. Med. 293 (24): 1255–7. doi:10.1056/NEJM197512112932412. PMID 1186807.
- ^ Parks DJ, Blanchard SG, Bledsoe RK, et al. (May 1999). "Bile acids: natural ligands for an orphan nuclear receptor". Science. 284 (5418): 1365–8. Bibcode:1999Sci...284.1365P. doi:10.1126/science.284.5418.1365. PMID 10334993.
- ^ Shin, DJ; Wang, L (2019). Bile Acid-Activated Receptors: A Review on FXR and Other Nuclear Receptors. Handbook of Experimental Pharmacology. Vol. 256. pp. 51–72. doi:10.1007/164_2019_236. ISBN 978-3-030-22004-4. PMID 31230143. S2CID 195327087.
- ^ Thistle JL, Hofmann AF (September 1973). "Efficacy and specificity of chenodeoxycholic acid therapy for dissolving gallstones". N. Engl. J. Med. 289 (13): 655–9. doi:10.1056/NEJM197309272891303. PMID 4580472.
- ^ a b Hofmann, AF (September 1989). "Medical dissolution of gallstones by oral bile acid therapy". American Journal of Surgery. 158 (3): 198–204. doi:10.1016/0002-9610(89)90252-3. PMID 2672842.
- ^ Berginer VM, Salen G, Shefer S (December 1984). "Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid". N. Engl. J. Med. 311 (26): 1649–52. doi:10.1056/NEJM198412273112601. PMID 6504105.
- ^ Broughton, G II (January 1994). "Chenodeoxycholate: the bile acid. The drug. a review". The American Journal of the Medical Sciences. 307 (1): 54–63. doi:10.1097/00000441-199401000-00011. PMID 8291509.
- ^ Rao, AS; Wong, BS; Camilleri, M; Odunsi-Shiyanbade, ST; McKinzie, S; Ryks, M; Burton, D; Carlson, P; Lamsam, J; Singh, R; Zinsmeister, AR (November 2010). "Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis". Gastroenterology. 139 (5): 1549–58, 1558.e1. doi:10.1053/j.gastro.2010.07.052. PMC 3189402. PMID 20691689.
- ^ Giaconda (8 July 2008). "Giaconda Suspends Hepaconda Phase II Trial to Carry Out Dose Ranging". Fierce Biotech (Press release). Sydney, Australia. Archived from the original on 7 April 2014. Retrieved 5 April 2014.