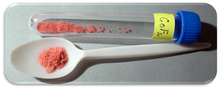
Cobalt(II) fluoride is a chemical compound with the formula (CoF2). It is a pink crystalline solid compound[1][2] which is antiferromagnetic at low temperatures (TN=37.7 K)[3] The formula is given for both the red tetragonal crystal, (CoF2), and the tetrahydrate red orthogonal crystal, (CoF2·4H2O). CoF2 is used in oxygen-sensitive fields, namely metal production. In low concentrations, it has public health uses. CoF2 is sparingly soluble in water. The compound can be dissolved in warm mineral acid, and will decompose in boiling water. Yet the hydrate is water-soluble, especially the di-hydrate CoF2·2H2O and tri-hydrate CoF2·3H2O forms of the compound. The hydrate will also decompose with heat.
| |
| |
| Names | |
|---|---|
| IUPAC name
Cobalt(II) fluoride
| |
| Other names
cobalt difluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.044 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CoF2 | |
| Molar mass | 96.93 g/mol |
| Appearance | Red crystalline solid |
| Density | 4.46 g/cm3 (anhydrous) 2.22 g/cm3 (tetrahydrate) |
| Melting point | 1,217 °C (2,223 °F; 1,490 K) |
| Boiling point | 1,400 °C (2,550 °F; 1,670 K) |
| 1.4 g/100 mL (25 °C) | |
| Solubility | soluble in HF insoluble in alcohol, ether, benzene |
| +9490.0·10−6 cm3/mol | |
| Structure | |
| tetragonal (anhydrous) orthorhombic (tetrahydrate) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
oral (rat): 150 mg/kg |
| Related compounds | |
Other anions
|
cobalt(II) oxide, cobalt(II) chloride |
Other cations
|
iron(II) fluoride, nickel(II) fluoride |
Related compounds
|
cobalt trifluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

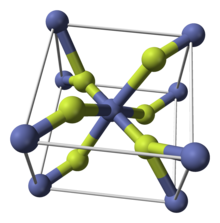
Like some other metal difluorides, CoF2 crystallizes in the rutile structure, which features octahedral Co centers and planar fluorides.[4]
Preparation
editCobalt(II) fluoride can be prepared from anhydrous cobalt(II) chloride or cobalt(II) oxide in a stream of hydrogen fluoride:
- CoCl2 + 2HF → CoF2 + 2HCl
- CoO + 2HF → CoF2 + H2O
It is produced in the reaction of cobalt (III) fluoride with water.
The tetrahydrate cobalt(II) fluoride is formed by dissolving cobalt(II) in hydrofluoric acid. The anhydrous fluoride can be extracted from this by dehydration. Other synthesis can occur at higher temperatures. It has been shown that at 500 °C fluorine will combine with cobalt producing a mixture of CoF2 and CoF3.[5]
Uses
editCobalt(II) fluoride can be used as a catalyst to alloy metals. It is also used for optical deposition, of which it tremendously improves optical quality. Cobalt(II) fluoride is available in most volumes in an ultra high purity composition. High purity compositions improve optical qualities and its usefulness as a standard.
Analysis
editTo analyze this compound, Cobalt (II) fluoride can be dissolved in nitric acid. The solution is then diluted with water until appropriate concentration for AA or ICP spectrophotometry for the cobalt. A small amount of salt can be dissolved in cold water and analyzed for fluoride ion by a fluoride ion-selective electrode or ion chromatography.
Chemical properties
editCoF2 is a weak Lewis acid. Cobalt(II) complexes are usually octahedral or tetrahedral. As a 19-electron species it is a good reducing agent, fairly oxidizable into an 18-electron compound. Cobalt(II) fluoride can be reduced by hydrogen at a 300 °C.
References
edit- ^ Pradyot Patnaik (2002), Handbook of Inorganic Chemicals, McGraw-Hill Professional, ISBN 978-0-07-049439-8
- ^ Pashkevich, D. S.; Radchenko S. M.; Mukhortov, D. A., "Article title Heat Exchange between Cobalt(II) Fluoride Powder and the Wall of Rotating Cylinder" (PDF), Russian Journal of Applied Chemistry, Consultants Bureau, ISSN 1070-4272, archived from the original (PDF) on 2004-09-29, retrieved 2007-03-07
- ^ Ashcroft/Mermin: Solid State Physics (Tab. 33.2)
- ^ Stout, J. W.; Reed, Stanley A. (1954). "The Crystal Structure of MnF2, FeF2, CoF2, NiF2 and ZnF2". J. Am. Chem. Soc. 76 (21): 5279–5281. doi:10.1021/ja01650a005.
- ^ J.C. Bailar (1973), Comprehensive Inorganic Chemistry, Pergoamon
