Lysergic acid hydroxyethylamide
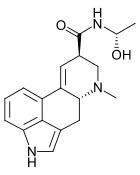
D-Lysergic acid α-hydroxyethylamide (LSH, LAH), also known as D-lysergic acid methyl carbinolamide, is a Lysergamide and alkaloid of the Ergoline family, it is present in higher concentrations in the parasitic fungi species "Claviceps", mainly the Claviceps paspali, also in Claviceps Purpurea. This fungi grows in various species in the Convolvulaceae family like the Ipomoea violacea (Heavenly Blue Morning Glory), the Rivea corymbosa (Ololiuhqui), and the Argyreia nervosa (Hawaiian Baby Woodrose). Heavenly Blue Morning Glory and Hawaiian Baby Woodrose especially contain high amounts of LSH, with content varying between species and by how fresh the seeds are. LSH is a psychoactive Ergoline and has effects similar to LSD due to similarity in the structure and is the main psychoactive compound found in Claviceps Paspali and in (fresh) Heavenly Blue Morning Glory Seeds. LSH is unstable and breaks down into LSA quickly, so old seeds often only contains LSA and iso-LSA. When the seeds are fresh, they contain significantly higher amounts of LSH.
 | |
| Clinical data | |
|---|---|
| Other names | D-lysergic acid methyl carbinolamide |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.079 |
| Chemical and physical data | |
| Formula | C18H21N3O2 |
| Molar mass | 311.385 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Chemistry
editThe structure is similar to LSD, with the N,N- diethylamide group replaced by an N- (1- hydroxyethyl)amide in D-lysergic acid α-hydroxyethylamide.
Pharmacology
editLSH has an affinity for several dopamine and serotonin receptors, mainly the 5-HT2 receptor like most psychedelics in the human brain. LSD is a partial agonist on many dopamine and serotonin receptors, so it is highly likely that LSH is also an agonist at dopamine and serotonin receptors.
[...] the new naturally occurring alkaloid D-lysergic acid methyl carbinolamide has powerful ergometrine-like oxytocic action and weak ergotamine-like adrenergic blocking actions. It must be included, on the basis of pharmacological evidence, in the ergometrine group of ergot alkaloids. Ergometrine, however, is less toxic and more active than the new alkaloid. Results suggest that it could have a lysergic acid diethylamide-like activity, but this hypothesis must be checked by experiments on humans.[2]
— Glasser, A.
Effects
editOne of the alkaloids in the seeds of Rivea corymbosa (Ololiuhqui), Argyreia nervosa (Hawaiian Baby Woodrose), and Ipomoea violacea (Tlitliltzin) are ergine (LSA) and isoergine (its epimer).[3]D-lysergic acid α-hydroxyethylamide is very limited/undocumented in human studies.[3]
LSH side effects include sedation, nausea, stimulation, hallucinations (similar to LSD), and euphoria. The headspace is described by many as feeling drunk.
Legality
editD-lysergic acid α-hydroxyethylamide is unscheduled and uncontrolled in the United States, but possession and sales of it for human consumption could potentially be prosecuted under the Federal Analog Act because of its structural similarities to LSD. Although doubtful as it breaks down into LSA which is a Schedule 3 drug and therefore not applicable to the Federal Analog Act.
See also
edit- Ergoline
- Lysergic acid
- LSA
- LSD
- Ergot
- Hawaiian baby woodrose (Argyreia nervosa)
- Ololiuhqui (Rivea corymbosa)
- Tlitliltzin (Ipomoea violacea)[verification needed][citation needed]
References
edit- ^ "Arrêté du 20 mai 2021 modifiant l'arrêté du 22 février 1990 fixant la liste des substances classées comme stupéfiants". www.legifrance.gouv.fr (in French). 20 May 2021.
- ^ Glasser A (January 1961). "Some pharmacological actions of D-lysergic acid methyl carbinolamide". Nature. 189 (4761): 313–4. Bibcode:1961Natur.189..313G. doi:10.1038/189313a0. PMID 13705953. S2CID 4260358.
- ^ a b Hofmann A (1971). "Teonanácatl and Ololiuqui, two ancient magic drugs of Mexico". Bulletin on Narcotics. 1: 3–14.
External links
edit- Ergot - A Rich Source of Pharmacologically Active Substances by Albert Hofmann