Subacute sclerosing panencephalitis (SSPE), also known as Dawson disease, is a rare form of progressive brain inflammation caused by a persistent infection with the measles virus. The condition primarily affects children, teens, and young adults. It has been estimated that about 2 in 10,000 people who get measles will eventually develop SSPE.[1] However, a 2016 study estimated that the rate for unvaccinated infants under 15 months was as high as 1 in 609.[2][3] No cure for SSPE exists, and the condition is almost always fatal. SSPE should not be confused with acute disseminated encephalomyelitis, which can also be caused by the measles virus, but has a very different timing and course.[4]
| Subacute sclerosing panencephalitis | |
|---|---|
| Other names | Dawson disease |
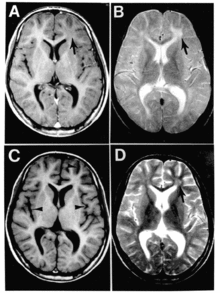 | |
| Subacute sclerosing panencephalitis. | |
| Specialty | Neurology, Infectious Disease |
| Symptoms | Behavior changes, seizures, spasticity, poor coordination, coma |
| Usual onset | 6-15 years after infection with measles |
| Causes | Measles virus |
| Risk factors | Measles infection |
| Diagnostic method | EEG, Serologic testing, brain biopsy |
| Prevention | Measles vaccine |
| Treatment | Supportive treatment |
| Medication | Intrathecal interferon alpha, intravenous ribavirin, isoprinosine |
| Prognosis | Usually fatal |
| Frequency | 2 in 10,000 for all age groups;[1] as high as 1 in 609 for unvaccinated infants under 15 months[2] |
SSPE is caused by the wild-type virus, not by vaccine strains.[5][6]
Signs and symptoms
editSSPE is characterized by a history of primary measles infection, followed by an asymptomatic period that lasts 7 years on average but can range from 1 month to 27 years. After the asymptomatic period, progressive neurological deterioration occurs, characterized by behavior change, intellectual problems, myoclonic seizures, blindness, ataxia, and eventually death.[7][8]
Stages of progression
editSymptoms progress through the following 4 stages:[9][10]
- Stage 1: There may be personality changes, mood swings, or depression. Fever, headache, and memory loss may also be present. This stage may last up to 6 months.
- Stage 2: This stage may involve jerking, muscle spasms, seizures, loss of vision, and dementia.
- Stage 3: Jerking movements are replaced by writhing (twisting) movements and rigidity. At this stage, complications may result in blindness or death.
- Stage 4: Progressive loss of consciousness into a persistent vegetative state, which may be preceded by or concomitant with paralysis, occurs in the final stage, during which breathing, heart rate, and blood pressure are affected. Death usually occurs as a result of fever, heart failure, or the brain’s inability to control the autonomic nervous system.
Pathogenesis
editA large number of nucleocapsids are produced in the neurons and the glial cells. In these cells the viral genes that encode envelope proteins have restricted expression.[11] As a result, infectious particles like the M protein are not produced, and the virus is able to survive persistently without evoking an immune response. Eventually the infection will lead to SSPE.[12]
Diagnosis
editAccording to the Merck Manual:[8]
"SSPE is suspected in young patients with dementia and neuromuscular irritability. EEG, CT or MRI, CSF examination, and measles serologic testing are done. EEG shows periodic complexes with high-voltage diphasic waves occurring synchronously throughout the recording. CT or MRI may show cortical atrophy or white matter lesions. CSF examination usually reveals normal pressure, cell count, and total protein content; however, CSF globulin is almost always elevated, constituting up to 20 to 60% of CSF protein. Serum and CSF contain elevated levels of measles virus antibodies. Anti-measles IgG appears to increase as the disease progresses. If test results are inconclusive, brain biopsy may be needed."
Treatment
editThere is no cure available.[13] If the diagnosis is made during stage 1 it may be possible to treat the disease with oral isoprinosine (Inosiplex) and intraventricular interferon alfa, but the response to these drugs varies from patient to patient,[14] and the only accepted treatments are supportive measures such as anticonvulsants.[8] Following onset of stage 2, the disease is invariably fatal.
Prognosis
editIn the classic presentation of the disease, death occurs in 1 to 3 years,[15] but faster and slower progressions can occur. Faster deterioration in cases of acute fulminant SSPE leads to death within 3 months of diagnosis.[16][17] Although the prognosis is bleak for SSPE past stage 1, there is a 5% spontaneous remission rate—this may be either a full remission that may last many years or an improvement in condition giving a longer progression period or at least a longer period with the less severe symptoms.[17][18]
Epidemiology
editSSPE is a rare condition, although there is still relatively high incidence in Asia and the Middle East. However, the number of reported cases is declining since the introduction of the measles vaccine—eradication of the measles virus prevents the SSPE mutation and therefore the progression of the disease or even the initial infection itself.[citation needed]
References
edit- ^ a b Bellini WJ, Rota JS, Lowe LE, Katz RS, Dyken PR, Zaki SR, Shieh WJ, Rota PA (2005). "Subacute sclerosing panencephalitis: more cases of this fatal disease are prevented by measles immunization than was previously recognized". The Journal of Infectious Diseases. 192 (10): 1686–1693. doi:10.1086/497169. PMID 16235165.
- ^ a b Wendorf, K, Winter, K, Harriman, K, Zipprich, J, Schechter, R, Hacker, J, Preas, C, Cherry JD, Glaser, C (2016). "Subacute Sclerosing Panencephalitis: The Devastating Measles Complication Is More Common Than We Think". Open Forum Infectious Diseases. 3. doi:10.1093/ofid/ofw194.81.
- ^ Sun, Lena (October 28, 2016). "New data shows a deadly measles complication is more common than thought". The Washington Post. Retrieved October 28, 2016.
- ^ Fisher DL, Defres S, Solomon T (2015). "Measles-induced encephalitis". QJM. 108 (3): 177–182. doi:10.1093/qjmed/hcu113. PMID 24865261.
- ^ Jafri, Sidra K; Kumar, Raman; Ibrahim, Shahnaz H (2018-06-26). "Subacute sclerosing panencephalitis – current perspectives". Pediatric Health, Medicine and Therapeutics. 9: 67–71. doi:10.2147/PHMT.S126293. ISSN 1179-9927. PMC 6027681. PMID 29985487.
- ^ Campbell, H; Andrews, N; Brown, K E; Miller, E (2007). "Review of the effect of measles vaccination on the epidemiology of SSPE". International Journal of Epidemiology. 36 (6): 1334–1348. doi:10.1093/ije/dym207. PMID 18037676.
- ^ "CDC pinkbook". 2019-03-29.
- ^ a b c "merckmanuals.com".
- ^ "medline.gov".
- ^ National Institute of Neurological Disorders and Stroke (NINDS) (27 March 2019). "Subacute Sclerosing Panencephalitis Information Page | National Institute of Neurological Disorders and Stroke". ninds.nih.gov. Retrieved 21 November 2021.
The initial symptoms of SSPE are subtle and include mild mental deterioration (such as memory loss) and changes in behavior (such as irritability) followed by disturbances in motor function, including uncontrollable involuntary jerking movements of the head, trunk or limbs called myoclonic jerks. Seizures may also occur. Some people may become blind. In advanced stages of the disease, individuals may lose the ability to walk, as their muscles stiffen or spasm. There is progressive deterioration to a comatose state, and then to a persistent vegetative state. Death is usually the result of fever, heart failure, or the brain's inability to continue controlling the autonomic nervous system.
- ^ Jawetz. Melnick & Adelberg's Medical Microbiology. Lange. 2010. p. 586. ISBN 978-0-07-174271-9.
- ^ Carter, M. J.; Willcocks, M. M.; Ter Meulen, V. (1983). "Defective translation of measles virus matrix protein in a subacute sclerosing panencephalitis cell line". Nature. 305 (5930): 153–5. Bibcode:1983Natur.305..153C. doi:10.1038/305153a0. PMC 7094927. PMID 6888557.
- ^ Rocke, Zoe; Belyayeva, Mariya (2022), "Subacute Sclerosing Panencephalitis", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 32809508, retrieved 2023-03-10
- ^
- Gascon G, Yamanis S, Crowell J, et al. Combined oralisoprinosine-intraventricular alpha-interferon therapy for subacute sclerosing panencephalitis. Brain Dev. 1993; 15:346–55.
- Anlar B, Yalaz K, Oktem F; et al. (1997). "Long-term follow-up of patients with subacute sclerosing panencephalitis treated with intraventricular alpha-interferon". Neurology. 48 (2): 526–8. doi:10.1212/wnl.48.2.526. PMID 9040751. S2CID 20412574.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Cianchetti C, Marrosu MG, Muntoni F; et al. (1998). "Intraventricularalpha-interferon in subacute sclerosing panencephalitis". Neurology. 50 (1): 315–16. doi:10.1212/wnl.50.1.315. PMID 9443512. S2CID 33700234.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
- ^ "SSPE information page www.ninds.nih.gov".
- ^ Risk WS, Haddad FS (1979). "The variable natural history of subacute sclerosing panencephalitis: a study of 118 cases from the Middle East". Arch Neurol. 56 (10): 610–14. doi:10.1001/archneur.1979.00500460044004. PMID 485888.
- ^ a b Garg, R K (1 February 2002). "Subacute sclerosing panencephalitis". Postgraduate Medical Journal. 78 (916): 63–70. doi:10.1136/pmj.78.916.63. PMC 1742261. PMID 11807185.
- ^
- Grunewald T, Lampe J, Weissbrich B; et al. (1998). "A 35-year old bricklayer with hemimyoclonic jerks". Lancet. 351 (9120): 1926. doi:10.1016/s0140-6736(98)04091-4. PMID 9654261. S2CID 206010725.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Santoshkumar B, Radhakrishnan K (1998). "Substantial spontaneous long-term remission in subacute sclerosing panencephalitis (SSPE)". J Neurol Sci. 154 (1): 83–8. doi:10.1016/s0022-510x(97)00303-1. PMID 9543327. S2CID 10635605.
- Grunewald T, Lampe J, Weissbrich B; et al. (1998). "A 35-year old bricklayer with hemimyoclonic jerks". Lancet. 351 (9120): 1926. doi:10.1016/s0140-6736(98)04091-4. PMID 9654261. S2CID 206010725.
Further reading
edit- Bonthius D, Stanek N, Grose C (2000). "Subacute sclerosing panencephalitis, a measles complication, in an internationally adopted child". Emerg Infect Dis. 6 (4): 377–81. doi:10.3201/eid0604.000409. PMC 2640885. PMID 10905971.
External links
edit- 23-273l. at Merck Manual of Diagnosis and Therapy Home Edition
- Subacute Sclerosing Panencephalitis at NINDS
- PubMed Health: Subacute sclerosing panencephalitis Archived 2014-08-08 at the Wayback Machine
- synd/1889 at Who Named It?