Aromatic L-amino acid decarboxylase inhibitor
An aromatic L-amino acid decarboxylase inhibitor (synonyms: DOPA decarboxylase inhibitor, extracerebral decarboxylase inhibitor, DDCI and AAADI) is a medication of type enzyme inhibitor which inhibits the synthesis of dopamine by the enzyme aromatic L-amino acid decarboxylase (AADC, AAAD, or DOPA decarboxylase). It is used to inhibit the decarboxylation of L-DOPA to dopamine outside the brain, i.e. in the blood. This is primarily co-administered with L-DOPA to combat Parkinson's disease. Administration can prevent common side-effects, such as nausea and vomiting, as a result of interaction with D2 receptors in the vomiting center (or cheomoreceptor trigger zone) located outside the blood–brain barrier.[2]
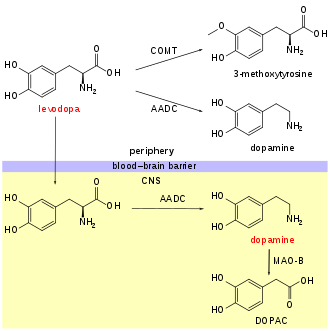
Examples of extracerebral decarboxylase inhibitors include carbidopa and benserazide.
Indications
editPeripherally selective DDCIs incapable of crossing the protective blood–brain barrier (BBB) are used in augmentation of L-DOPA (levodopa) in the treatment of Parkinson's disease (PD) to block the conversion of L-DOPA into dopamine outside the brain, for the purpose of reducing adverse side effects.[3] Combined L-DOPA and DDCI therapy does not inherently decrease peripheral cardiovascular side effects of L-DOPA administration; however, combined therapy potentiates the central effects of L-DOPA by decreasing the dose-dependency 4-5 fold, therein allowing for effective Parkinson's disease treatment without cardiovascular risk associated with high peripheral dopamine.[4][5]
List of DDCIs
edit- Benserazide (Madopar, Prolopa, Modopar, Madopark, Neodopasol, EC-Doparyl, etc.)
- Carbidopa (Lodosyn, Sinemet, Pharmacopa, Atamet, Stalevo, etc.)
- Methyldopa (Aldomet, Aldoril, Dopamet, Dopegyt, etc.)
- alpha-Difluoromethyl-DOPA (DFMD)
- 3',4',5,7-Tetrahydroxy-8-methoxyisoflavone [58262-89-8]
- Epigallocatechin gallate (EGCG)
- Epigallocatechin (EGC)
References
edit- ^ Mutschler, Ernst; Schäfer-Korting, Monika (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. pp. 313–316. ISBN 3-8047-1763-2.
- ^ Calne, D. B.; Reid, J. L.; Vakil, S. D.; Rao, S.; Petrie, A.; Pallis, C. A.; Gawler, J.; Thomas, P. K.; Hilson, A. (1971). "Idiopathic Parkinsonism treated with an extracerebral decarboxylase inhibitor in combination with levodopa". British Medical Journal. 3 (5777): 729–732. doi:10.1136/bmj.3.5777.729. PMC 1798919. PMID 4938431.
- ^ "Editorial: Dopa decarboxylase inhibitors". British Medical Journal. 4 (5939): 250–1. November 1974. doi:10.1136/bmj.4.5939.250. PMC 1612227. PMID 4425849.
- ^ Cotzias, G. C., Papavasiliou, P. S., and Gellene, R., New England Journal of Medicine, 1969, 280, 337.
- ^ Yahr, M. D., in Advances in Neurology, ed. M. D. Yahr, p. vi, vol. 2. New York, Raven Press, 1973.