Eplerenone, sold under the brand name Inspra, is an aldosterone antagonist type of potassium-sparing diuretic that is used to treat chronic heart failure and high blood pressure, particularly for people with resistant hypertension due to elevated aldosterone. It is a steroidal antimineralocorticoid of the spirolactone group and a selective aldosterone receptor antagonist (SARA).[6]
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɛpˈlɛrənoʊn/ |
| Trade names | Inspra, others |
| Other names | SC-66110; CGP-30083; 9-11α-Epoxymexrenone; 9,11α-Epoxy-7α-methoxycarbonyl-3-oxo-17α-pregn-4-ene-21,17-carbolactone |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603004 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~70%[2] |
| Protein binding | ~50% (33–60%) (primarily to α1-acid glycoprotein)[2][3] |
| Metabolism | Liver (CYP3A4)[2][3] |
| Metabolites | 6β-OH-EPL, 6β,21-OH-EPL, 21-OH-EPL, 3α,6β-OH-EPL[2] (All inactive)[2] |
| Elimination half-life | 4–6 hours[4] |
| Excretion | Urine (67%), feces (32%)[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.106.615 |
| Chemical and physical data | |
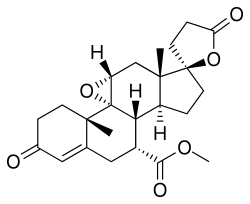
| Formula | C24H30O6 |
| Molar mass | 414.498 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Medical uses
editHeart failure
editEplerenone reduces risk of death in patients with heart failure,[7] particularly in patients with recent myocardial infarction (heart attack).[8]
Hypertension
editEplerenone lowers blood pressure in patients with primary hypertension.[9] Eplerenone also reduces arterial stiffness and vascular endothelial dysfunction.[10]
For persons with resistant hypertension, eplerenone is safe and effective for reducing blood pressure,[11] particularly in persons with resistant hypertension due to hyperaldosteronism.[12][13]
Central serous chorioretinopathy
editEplerenone is often prescribed for people with central serous chorioretinopathy (CSC). However, the most recent and largest randomized controlled trial showed that eplerenone has no significant effect on chronic CSC that has been untreated for four months.[14][15] There was one relatively large prospective, interventional case-control study that was tested in acute CSC that showed improved resolution of subretinal fluid in treatment group vs observational group (which is standard of care) with 45% resolution at end of 1st month, 55% at end of 2nd month, and 62% at end of 3rd month (vs 10%, 21%, and 31% in standard of care group). Study also showed faster resolution of visual acuity at the end of each month with 92% and 100% in the first two months vs 74% and 86% with resolution reaching 100% after the third month in standard of care group.[16]
Adverse effects
editCommon adverse drug reactions (ADRs) associated with the use of eplerenone include: hyperkalaemia, hypotension, dizziness, and reduced renal clearance.[17] Eplerenone may have a lower incidence than spironolactone of sexual side effects such as feminization, gynecomastia, impotence, low sex drive and reduction of size of male genitalia.[18] This is because other antimineralocorticoids have structural elements of the progesterone molecule, causing progestogenic and antiandrogenic outcomes.[4] When considering taking these medicines, it is important to note the variations in their ability to offset the nongenomic effects of aldosterone.[4]
Currently, there is not enough evidence available from the randomized controlled trials on side effects of eplerenone to do a benefit versus risk assessment in people with primary hypertension.[19]
Interactions
editEplerenone is primarily metabolized by the cytochrome P450 enzyme CYP3A4. Thus the potential exists for adverse drug interactions with other drugs that induce or inhibit CYP3A4. Specifically, the concomitant use of the CYP3A4 potent inhibitors ketoconazole and itraconazole is contraindicated. Other CYP3A4 inhibitors including erythromycin, saquinavir, and verapamil should be used with caution. Other drugs that increase potassium concentrations may increase the risk of hyperkalemia associated with eplerenone therapy, including salt substitutes,[20] potassium supplements and other potassium-sparing diuretics.
Pharmacology
editEplerenone is an antimineralocorticoid, or an antagonist of the mineralocorticoid receptor (MR).[21] Eplerenone is also known chemically as 9,11α-epoxy-7α-methoxycarbonyl-3-oxo-17α-pregn-4-ene-21,17-carbolactone and "was derived from spironolactone by the introduction of a 9α,11α-epoxy bridge and by substitution of the 17α-thoacetyl group of spironolactone with a carbomethoxy group."[22] The drug controls high blood pressure by blocking the binding of aldosterone to the mineralocorticoid receptor (MR) in epithelial tissues, such as the kidney.[4] Blocking the action of aldosterone decreases blood volume and lowers blood pressure.[23] It has 10- to 20-fold lower affinity for the MR relative to spironolactone,[21] and is less potent in vivo as an antimineralocorticoid.[4] However, in contrast to spironolactone, eplerenone has little affinity for the androgen, progesterone, and glucocorticoid receptors.[21][4] It also has more consistently observed non-genomic antimineralocorticoid effects relative to spironolactone (see membrane mineralocorticoid receptor).[4] Eplerenone differs from spironolactone in its extensive metabolism, with a short half-life and inactive metabolites.[4]
Eplerenone seems to be about 50 to 75% as potent as spironolactone as an antimineralocorticoid.[24] Hence, 25 mg/day spironolactone may be equivalent to approximately 50 mg/day eplerenone.[25]
Society and culture
editEplerenone was patented in 1983 and approved for medical use in the United States in 2002.[26][23] Eplerenone is approved for sale in Canada, the US, the EU, Netherlands, and Japan.[23]
Economics
editEplerenone costs an estimated $2.93 per day when treating congestive heart failure and $5.86 per day when treating hypertension.[18]
Brand names
editIn the US, Inspra is marketed by Viatris after Upjohn was spun off from Pfizer.[27][28][29]
References
edit- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ a b c d e Lemke TL, Williams DA (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 743–. ISBN 978-1-60913-345-0.
- ^ a b Sica DA (January 2005). "Pharmacokinetics and pharmacodynamics of mineralocorticoid blocking agents and their effects on potassium homeostasis". Heart Failure Reviews. 10 (1): 23–9. doi:10.1007/s10741-005-2345-1. PMID 15947888. S2CID 21437788.
- ^ a b c d e f g h Struthers A, Krum H, Williams GH (April 2008). "A comparison of the aldosterone-blocking agents eplerenone and spironolactone". Clinical Cardiology. 31 (4): 153–8. doi:10.1002/clc.20324. PMC 6652937. PMID 18404673.
- ^ Frishman WH, Cheng-Lai A, Nawarskas J (4 January 2005). Current Cardiovascular Drugs. Springer Science & Business Media. pp. 246–. ISBN 978-1-57340-221-7.
- ^ Delyani JA, Rocha R, Cook CS, Tobert DS, Levin S, Roniker B, et al. (2001). "Eplerenone: a selective aldosterone receptor antagonist (SARA)". Cardiovascular Drug Reviews. 19 (3): 185–200. doi:10.1111/j.1527-3466.2001.tb00064.x. PMID 11607037.
- ^ Frankenstein L, Seide S, Täger T, Jensen K, Fröhlich H, Clark AL, et al. (March 2020). "Relative Efficacy of Spironolactone, Eplerenone, and cAnRenone in patients with Chronic Heart failure (RESEARCH): a systematic review and network meta-analysis of randomized controlled trials". Heart Fail Rev. 25 (2): 161–171. doi:10.1007/s10741-019-09832-y. PMID 31364027. S2CID 198999728.
- ^ De Luca L (October 2020). "Established and Emerging Pharmacological Therapies for Post-Myocardial Infarction Patients with Heart Failure: a Review of the Evidence". Cardiovasc Drugs Ther. 34 (5): 723–735. doi:10.1007/s10557-020-07027-4. PMID 32564304. S2CID 219936888.
- ^ Tam TS, Wu MH, Masson SC, Tsang MP, Stabler SN, Kinkade A, et al. (February 2017). "Eplerenone for hypertension". The Cochrane Database of Systematic Reviews. 2017 (2): CD008996. doi:10.1002/14651858.CD008996.pub2. PMC 6464701. PMID 28245343.
- ^ Sakima A, Arima H, Matayoshi T, Ishida A, Ohya Y (March 2021). "Effect of Mineralocorticoid Receptor Blockade on Arterial Stiffness and Endothelial Function: A Meta-Analysis of Randomized Trials". Hypertension. 77 (3): 929–937. doi:10.1161/HYPERTENSIONAHA.120.16397. PMID 33461316.
- ^ Dahal K, Kunwar S, Rijal J, Alqatahni F, Panta R, Ishak N, et al. (November 2015). "The Effects of Aldosterone Antagonists in Patients With Resistant Hypertension: A Meta-Analysis of Randomized and Nonrandomized Studies". American Journal of Hypertension. 28 (11): 1376–85. doi:10.1093/ajh/hpv031. PMID 25801902.
- ^ Morimoto S, Ichihara A (August 2020). "Management of primary aldosteronism and mineralocorticoid receptor-associated hypertension". Hypertens Res. 43 (8): 744–753. doi:10.1038/s41440-020-0468-3. PMID 32424201. S2CID 218670505.
- ^ Spence JD (May 2017). "Rational Medical Therapy Is the Key to Effective Cardiovascular Disease Prevention". The Canadian Journal of Cardiology. 33 (5): 626–634. doi:10.1016/j.cjca.2017.01.003. PMID 28449833.
- ^ "Eplerenone does not improve vision in people with central serous chorioretinopathy". NIHR Evidence (Plain English summary). National Institute for Health and Care Research. 25 March 2020. doi:10.3310/signal-000893. S2CID 241440498.
- ^ Lotery A, Sivaprasad S, O'Connell A, Harris RA, Culliford L, Ellis L, et al. (January 2020). "Eplerenone for chronic central serous chorioretinopathy in patients with active, previously untreated disease for more than 4 months (VICI): a randomised, double-blind, placebo-controlled trial". Lancet. 395 (10220): 294–303. doi:10.1016/S0140-6736(19)32981-2. hdl:1983/c40782ba-12d7-428b-8724-bbffb45d7bcf. PMID 31982075.
- ^ Venkatesh R, Pereira A, Jayadev C, Prabhu V, Aseem A, Jain K, et al. (July 2020). "Oral Eplerenone Versus Observation in the Management of Acute Central Serous Chorioretinopathy: A Prospective, Randomized Comparative Study". Pharmaceuticals. 13 (8): 170. doi:10.3390/ph13080170. PMC 7463844. PMID 32751370.
- ^ Rossi S (2006). Australian Medicines Handbook 2006. Australian Medicines Handbook. ISBN 978-0-9757919-2-9.
- ^ a b Craft J (April 2004). "Eplerenone (Inspra), a new aldosterone antagonist for the treatment of systemic hypertension and heart failure". Proceedings. 17 (2): 217–20. doi:10.1080/08998280.2004.11927973. PMC 1200656. PMID 16200104.
- ^ Tam TS, Wu MH, Masson SC, Tsang MP, Stabler SN, Kinkade A, et al. (February 2017). "Eplerenone for hypertension". The Cochrane Database of Systematic Reviews. 2017 (2): CD008996. doi:10.1002/14651858.CD008996.pub2. PMC 6464701. PMID 28245343.
- ^ LoSalt Advisory Statement Archived 10 December 2005 at the Wayback Machine (PDF)
- ^ a b c Delyani JA (April 2000). "Mineralocorticoid receptor antagonists: the evolution of utility and pharmacology". Kidney International. 57 (4): 1408–11. doi:10.1046/j.1523-1755.2000.00983.x. PMID 10760075.
- ^ Brown NJ (May 2003). "Eplerenone: cardiovascular protection". Circulation. 107 (19): 2512–8. doi:10.1161/01.CIR.0000071081.35693.9A. PMID 12756192. S2CID 13904574.
- ^ a b c "Inspra (Eplerenone)". Drug Development Technology. Retrieved 19 April 2016.
- ^ Struthers A, Krum H, Williams GH (April 2008). "A comparison of the aldosterone-blocking agents eplerenone and spironolactone". Clin Cardiol. 31 (4): 153–8. doi:10.1002/clc.20324. PMC 6652937. PMID 18404673.
- ^ Peter L. Thompson (28 January 2011). Coronary Care Manual. Elsevier Health Sciences. pp. 254–. ISBN 978-0-7295-7927-8.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 459. ISBN 9783527607495.
- ^ "Pfizer Completes Transaction to Combine Its Upjohn Business with Mylan". Pfizer. 16 November 2020. Retrieved 17 June 2024 – via Business Wire.
- ^ "Inspra". Pfizer. Retrieved 17 June 2024.
- ^ "Brands". Viatris. 16 November 2020. Retrieved 17 June 2024.