Risdiplam, sold under the brand name Evrysdi, is a medication used to treat spinal muscular atrophy (SMA)[6][9] and the first oral medication approved to treat this disease.[6][9]
 | |
| Clinical data | |
|---|---|
| Trade names | Evrysdi |
| Other names | RG7916; RO7034067 |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.278.103 |
| Chemical and physical data | |
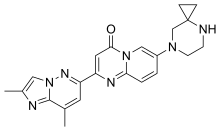
| Formula | C22H23N7O |
| Molar mass | 401.474 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Risdiplam is a survival of motor neuron 2-directed RNA splicing modifier.[6][5][10]
In clinical trials, the most common adverse events included fever, diarrhea, rash, ulcers of the mouth area, joint pain (arthralgia) and urinary tract infections.[6][5] Additional adverse events observed in the infantile-onset population included upper respiratory tract infection, pneumonia, constipation and vomiting.[6][5]
Risdiplam was approved by the US Food and Drug Administration (FDA) in August 2020, for the treatment of adults and children two months of age or older.[6][11] Developed by Roche in Basel, Switzerland,[12] in association with PTC Therapeutics and the SMA Foundation,[9][11] it is marketed in the US by Genentech,[6] a subsidiary of Roche.[11]
Medical uses
editIn the United States, risdiplam is indicated to treat people two months of age and older with spinal muscular atrophy.[6][5]
Adverse effects
editIn two clinical trials, the following adverse events occurred at least 5% more frequently in patients treated with risdiplam than in the placebo group: fever, diarrhoea, rash, ulcers of the mouth area, joint pain (arthralgia) and urinary tract infections.[6][5] Additional adverse events for the infantile-onset population included upper respiratory tract infection, pneumonia, constipation and vomiting.[6][5]
Risdiplam should not be taken together with medications that are multidrug and toxin extrusion (MATE) substrates because risdiplam may increase plasma concentrations of these drugs.[6][5]
Pharmacology
editMechanism of action
editRisdiplam addresses the underlying cause of SMA: a reduced amount of survival motor neuron (SMN) protein. The protein is encoded by the SMN1 and SMN2 genes. SMA is caused by mutations in SMN1 that code for inactive forms of the protein. The activity of the SMN2 gene, which produces much smaller quantities of SMN, tends to determine the severity of disease.[9][13]
The compound is a pyridazine derivative that modifies the splicing of SMN2 messenger RNA to include exon 7,[14][10][15] resulting in an increase in the concentration of the functional SMN protein in vivo.[12]
Nusinersen, the first drug approved to treat SMA, an anti-sense oligonucleotide targeting intronic splicing silencer N1 (ISS-N1), also alters mRNA splicing of SMN2.[16]
Efficacy
editThe safety and efficacy of risdiplam in infantile-onset and later-onset SMA has been evaluated in ongoing clinical trials.[9][17] [18]
In the infantile-onset SMA study, an open-label trial with 41 participants, efficacy was established based on the ability to sit without support for at least five seconds. After 12 months of treatment, 29% of participants were able to sit independently for more than five seconds. After 23 or more months of treatment, 81% of participants were alive without permanent ventilation. Although the study did not perform direct comparisons against children receiving a placebo (inactive treatment), these results compare favourably with the typical course of the untreated disease.[17][6]
The study of later-onset SMA was a randomised controlled trial that enrolled 180 participants, aged between 2 and 25 years, with less severe forms of the disease. Participants treated with risdiplam for 12 months showed improvements in motor function compared to participants given a placebo.[18][6][9]
Society and culture
editLegal status
editThe US Food and Drug Administration (FDA) awarded marketing approval to Genentech in August 2020. The FDA earlier granted the application for risdiplam fast track, priority review, and orphan drug designations.[6][9][11] Genentech was also awarded a rare pediatric disease priority review voucher.[6]
The European Medicines Agency (EMA) awarded risdiplam a priority medicine designation in 2018[11][19][20] and an orphan drug designation in 2019.[11][21]
As of August 2020[update], Roche has applied for marketing authorisation in Brazil, Chile, China, the European Union, Indonesia, Russia, South Korea and Taiwan.[11][22]
Names
editRisdiplam is the International nonproprietary name (INN).[23]
Compassionate use
editSince late 2019, Roche has been offering the drug globally for free to some eligible people through an expanded access program.[24]
References
edit- ^ a b "Evrysdi". Therapeutic Goods Administration (TGA). 11 June 2021. Retrieved 6 September 2021.
- ^ a b "AusPAR: Risdiplam". Therapeutic Goods Administration (TGA). 13 September 2021. Retrieved 13 September 2021.
- ^ "Summary Basis of Decision (SBD) for Evrysdi". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- ^ "Health product highlights 2021: Annexes of products approved in 2021". Health Canada. 3 August 2022. Retrieved 25 March 2024.
- ^ a b c d e f g h Food and Drugs Administration (FDA) (18 August 2020). "Evrysdi- risdiplam powder, for solution". DailyMed. Bethesda, Maryland, United States: MedLine by the National Library of Medicine (NLM) of the United States National Institutes of Health (NIH). Retrieved 24 September 2020.
- ^ a b c d e f g h i j k l m n o p O'Keefe L (7 August 2020). "FDA Approves Oral Treatment for Spinal Muscular Atrophy" (Press release). Silver Spring, Maryland, United States of America: United States Food and Drug Administration (FDA). FDA Newsroom Department. Archived from the original on 11 August 2020. Retrieved 7 August 2020. This article incorporates text from this source, which is in the public domain.
- ^ "Evrysdi EPAR". European Medicines Agency. 24 February 2021. Retrieved 4 March 2023.
- ^ "Evrysdi Product information". Union Register of medicinal products. Retrieved 3 March 2023.
- ^ a b c d e f g Food and Drugs Administration (FDA) (7 August 2020). "Evrysdi (Risdiplam) for Spinal Muscular Atrophy". SMA News Today. Pensacola, Florida, United States: BioNews Services (BioNews Services, LLC.). Archived from the original on 27 January 2021. Retrieved 9 June 2021.
- ^ a b Zhao X, Feng Z, Ling KK, Mollin A, Sheedy J, Yeh S, et al. (May 2016). "Pharmacokinetics, pharmacodynamics, and efficacy of a small-molecule SMN2 splicing modifier in mouse models of spinal muscular atrophy". Human Molecular Genetics. 25 (10). Oxford, United Kingdom of Great Britain: Oxford University Press (OUP): 1885–1899. doi:10.1093/hmg/ddw062. PMC 5062580. PMID 26931466.
- ^ a b c d e f g "FDA Approves Genentech's Evrysdi (risdiplam) for Treatment of Spinal Muscular Atrophy (SMA) in Adults and Children 2 Months and Older". Genentech (Press release). San Francisco, California, United States of America: Genentech, Inc. (Roche Group). Genentech Global Product Development Division. 7 August 2020. Archived from the original on 18 August 2020. Retrieved 7 August 2020.
- ^ a b Ratni H, Ebeling M, Baird J, Bendels S, Bylund J, Chen KS, et al. (August 2018). "Discovery of Risdiplam, a Selective Survival of Motor Neuron-2 ( SMN2) Gene Splicing Modifier for the Treatment of Spinal Muscular Atrophy (SMA)". Journal of Medicinal Chemistry. 61 (15). Washington, D.C.: 6501–6517. doi:10.1021/acs.jmedchem.8b00741. LCCN a63000643. OCLC 39480771. PMID 30044619.
- ^ Ramdas S, Servais L (February 2020). Grech D, Mikhailidis D, Abdollahi M (eds.). "New treatments in spinal muscular atrophy: an overview of currently available data". Expert Opinion on Pharmacotherapy. 21 (3). London, England, United Kingdom of Great Britain: Taylor & Francis (Informa UK Ltd): 307–315. doi:10.1080/14656566.2019.1704732. OCLC 57378019. PMID 31973611. S2CID 210880199.
- ^ "RG7916". Pensacola, Florida, United States: BioNews Services (BioNews Services, LLC.). 8 November 2016. Archived from the original on 24 April 2020. Retrieved 9 June 2021.
- ^ Baranello G, Darras BT, Day JW, Deconinck N, Klein A, Masson R, et al. (March 2021). "Risdiplam in Type 1 Spinal Muscular Atrophy". The New England Journal of Medicine. 384 (10). Boston, Massachusetts, United States of America: NEJM Group (Massachusetts Medical Society): 915–923. doi:10.1056/NEJMoa2009965. LCCN 20020456. OCLC 231027780. PMID 33626251. S2CID 232047598.
- ^ Zanetta C, Nizzardo M, Simone C, Monguzzi E, Bresolin N, Comi GP, Corti S (January 2014). "Molecular therapeutic strategies for spinal muscular atrophies: current and future clinical trials". Clinical Therapeutics. 36 (1). Philadelphia, Pennsylvania, United States of America: Elsevier: 128–140. doi:10.1016/j.clinthera.2013.11.006. PMID 24360800.
- ^ a b Baranello G, Servais L, Day J, Deconinck N, Mercuri E, Klein A, et al. (1 October 2019). "P.353FIREFISH Part 1: 16-month safety and exploratory outcomes of risdiplam (RG7916) treatment in infants with type 1 spinal muscular atrophy". Neuromuscular Disorders. 29 (Supplement 1). London, United Kingdom of Great Britain: World Muscle Society (WMS)/Elsevier Inc.: S184. doi:10.1016/j.nmd.2019.06.515. ISSN 0960-8966. OCLC 24318845.
- ^ a b Mercuri E, Baranello G, Kirschner J, Servais L, Goemans N, Pera MC, et al. (16 April 2019). "Update from SUNFISH Part 1: Safety, Tolerability and PK/PD from the Dose-Finding Study, Including Exploratory Efficacy Data in Patients with Type 2 or 3 Spinal Muscular Atrophy (SMA) Treated with Risdiplam (RG7916) (S25.007)". Neurology. 92 (15 (Supplement)). Minneapolis, Minnesota, United States of America: American Academy of Neurology/Wolters Kluwer. doi:10.1212/WNL.92.15_supplement.S25.007. ISSN 0028-3878. LCCN 55043902. OCLC 960771045. Retrieved 9 June 2021.
- ^ Inacio P (21 December 2018). "Risdiplam Granted EMA's PRIME Designation for Potential in Spinal Muscular Atrophy". SMA News Today. Pensacola, Florida, United States: BioNews Services (BioNews Services, LLC.). Archived from the original on 26 January 2021. Retrieved 9 June 2021.
- ^ Roche Group Media Relations Division (17 December 2018). "PRIME designation granted by European Medicines Agency for Roche's risdiplam for treatment of spinal muscular atrophy (SMA)" (Press release). Basel, Switzerland: F. Hoffmann-La Roche Ltd. Group Communications Department (Roche Group Media Relations Division). pp. 1–5. Archived from the original (PDF) on 18 August 2020. Retrieved 9 June 2021.
- ^ Stoyanova-Beninska V, Schwarzer-Daum B (26 February 2019). Public summary of opinion on orphan designation: Risdiplam for the treatment of spinal muscular atrophy (PDF). EMA Committee for Orphan Medicinal Products (COMP) (Report). Amsterdam, the Netherlands: European Medicines Agency (EMA Committee for Orphan Medicinal Products). pp. 1–4. Archived (PDF) from the original on 6 May 2020.
- ^ PTC Therapeutics (17 August 2020). "PTC Announces the Acceptance of the European Marketing Authorization Application for Evrysdi™ (risdiplam) for the Treatment of Spinal Muscular Atrophy" (Press release). Albuquerque, New Mexico, United States of America: PR Newswire. PTC Therapeutics. Archived from the original on 18 August 2020. Retrieved 9 June 2021.
- ^ World Health Organization (2018). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 80". WHO Drug Information. 32 (3): 482. hdl:10665/330907.
- ^ Petridis F (13 January 2020). "Roche announces global compassionate use programme for Risdiplam" (Press release). Stratford-upon-Avon, England, United Kingdom of Great Britain: Spinal Muscular Atrophy UK ltd. Roche Global SMA Team (F. Hoffmann-La Roche Ltd). Retrieved 9 June 2021.
Further reading
edit- Dhillon S (November 2020). "Risdiplam: First Approval". Drugs. 80 (17). Berlin, Germany/Heidelberg, Germany/Cham, Switzerland: Adis International/Springer Nature Switzerland AG (Springer Nature): 1853–1858. doi:10.1007/s40265-020-01410-z. OCLC 01566990. PMID 33044711. S2CID 222279898.
- Ratni H, Scalco RS, Stephan AH (June 2021). "Risdiplam, the First Approved Small Molecule Splicing Modifier Drug as a Blueprint for Future Transformative Medicines". ACS Medicinal Chemistry Letters. 12 (6). Washington, D.C.: ACS Publications (American Chemical Society): 874–877. doi:10.1021/acsmedchemlett.0c00659. OCLC 643819990. PMC 8201486. PMID 34141064.
External links
edit- Clinical trial number NCT02913482 for "Investigate Safety, Tolerability, PK, PD and Efficacy of Risdiplam (RO7034067) in Infants With Type1 Spinal Muscular Atrophy (FIREFISH)" at ClinicalTrials.gov
- Clinical trial number NCT02908685 for "A Study to Investigate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Efficacy of Risdiplam (RO7034067) in Type 2 and 3 Spinal Muscular Atrophy (SMA) Participants (SUNFISH)" at ClinicalTrials.gov