This is a list of fentanyl analogues (sometimes referred to as Fentalogs),[1][2][3] including both compounds developed by pharmaceutical companies for legitimate medical use, and those which have been sold as designer drugs and reported to national drug control agencies such as the DEA, or transnational agencies such as the EMCDDA and UNODC.[4][5][6][7][8][9][10] This is not a comprehensive listing of fentanyl analogues, as more than 1400 compounds from this family have been described in the scientific and patent literature,[11][12][13][14][15] but it includes many notable compounds that have reached late-stage human clinical trials, or which have been identified as having been sold as designer drugs, as well as representative examples of significant structural variations reported in the scientific and patent literature. The structural variations among fentanyl-related substances can impart profound pharmacological differences between these drugs, especially with respect to potency and efficacy.[16][17][18][19][20]
In the United States, the Drug Enforcement Administration placed the broadly defined class of "Fentanyl-Related Substances" on the list of Schedule I drugs in 2018, making it illegal to manufacture, distribute, or possess fentanyl analogs.[21] Temporary control of fentanyl-related substances in Schedule I was extended through December 31, 2024 by Public Law 117-328.[22]
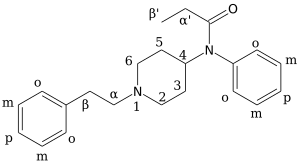
Chemical structures of various fentanyl analogues
edit| Chemical structure | Common name | Chemical name | CAS number |
|---|---|---|---|
| 2-Fluoroacrylfentanyl | N-(2-Fluorophenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-propen-2-amide | 2309383-09-1 | |
| 2-Fluorobutyrfentanyl (o-FBF) | N-(2-Fluorophenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-butanamide | 2163847-76-3 | |
| 2-Fluoroisobutyrfentanyl (o-FIBF) | N-(2-Fluorophenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-2-methylpropanamide | 2351142-33-9 | |
| 2-Methylacetylfentanyl (o-MAF) | N-(2-Methylphenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-acetamide | 90736-11-1 | |
| 2-Methylmethoxyacetylfentanyl | N-(2-Methylphenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-2-methoxyacetamide | ||
| 2,5-Dimethylfentanyl | N-[2,5-Dimethyl-1-(2-phenylethyl)piperidin-4-yl]-N-phenylpropanamide | 42045-97-6 | |
| 2,2'-Difluorofentanyl | N-(2-Fluorophenyl)-N-[1-(2-[2-fluorophenyl]ethyl)-4-piperidinyl]-propanamide | 2748343-87-3 | |
| 3-Allylfentanyl | N-[(3S,4R)-1-Phenethyl-3-prop-2-enylpiperidin-4-yl]-N-phenylpropanamide | 82208-84-2 | |
| 3-Fluorofentanyl (NFEPP) | N-(3-Fluoro-1-phenethylpiperidin-4-yl)-N-phenylpropionamide | 1422952-84-8 | |
| 3-Furanylfentanyl (3FUF) | N-Phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]furan-3-carboxamide | 101343-82-2 | |
| 3-Methylbutyrfentanyl | N-[3-Methyl-1-(2-phenylethyl)piperidin-4-yl]-N-phenylbutanamide | 97605-09-9 | |
| 3-Methylcrotonylfentanyl | N-Phenyl-N-[1-(2-phenylethyl)-4-(3-methylpiperidin-1-yl)]-2-butenamide | ||
| p-Methylcrotonylfentanyl | N-(4-methylphenyl)-N-[1-(2-phenylethyl)-4-(piperidin-1-yl)]-2-butenamide | ||
| 3-Methylfentanyl (3-MF) | N-(3-methyl-1-phenethyl-4-piperidyl)-N-phenyl-propanamide | 42045-86-3 | |
| 3-Methylfuranylfentanyl[23] (3MFUF, TMFUF) | N-Phenyl-N-[3-methyl-1-(2-phenylethyl)piperidin-4-yl]furan-2-carboxamide | ||
| 3-Methylthiofentanyl | N-{3-Methyl-1-[2-(2-thienyl)ethyl]piperidin-4-yl}-N-phenylpropanamide | 86052-04-2 | |
| 3-Phenylpropanoylfentanyl[24] | N-Phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]-3-phenylpropanamide | 79279-02-0 | |
| 4-Fluorobutyrfentanyl (4-FBF) | N-(4-Fluorophenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-butanamide | 244195-31-1 | |
| 4-Chloroisobutyrylfentanyl (4-CIBF) [25] | 2-Methyl-N-(4-chlorophenyl)-N-[1-(1-phenylpropan-2-yl)piperidin-4-yl]propanamide | 244195-34-4 | |
| 4-Fluoroisobutyrfentanyl (4-FIBF) | N-(4-Fluorophenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-isobutanamide | 244195-32-2 | |
| 4-Fluorofentanyl | N-(4-fluorophenyl)-N-[1-(2-phenylethyl)piperidin-4-yl]propanamide | 90736-23-5 | |
| Paramethylfentanyl (p-MF) | N-(4-methylphenyl)-N-[1-(2-phenylethyl)piperidin-4-yl]propanamide | 1838-67-1 | |
| para-fluorofuranylfentanyl[26] (p-F-Fu-F) | N-(1-phenethylpiperidin-4-yl)-N-(4-fluorophenyl)furan-2-carboxamide | 1802489-71-9 | |
| para-chlorofuranylfentanyl (p-Cl-Fu-F) | N-(1-phenethylpiperidin-4-yl)-N-(4-chlorophenyl)furan-2-carboxamide | ||
| ortho-methylfuranylfentanyl (o-Me-Fu-F) | N-(1-phenethylpiperidin-4-yl)-N-(o-tolyl)furan-2-carboxamide | 2309383-07-9 | |
| ortho-methoxyfuranylfentanyl (o-MeO-Fu-F) | N-(1-phenethylpiperidin-4-yl)-N-(2-methoxyphenyl)furan-2-carboxamide | 101343-50-4 | |
| ortho-isopropylfuranylfentanyl (o-iPr-Fu-F) | N-(1-phenethylpiperidin-4-yl)-N-(2-isopropylphenyl)furan-2-carboxamide | ||
| 4-Phenylfentanyl | N-Phenyl-N-[4-phenyl-1-(2-phenylethyl)piperidin-4-yl]propanamide | 120448-97-7 | |
| 4-Methoxybutyrfentanyl | N-(4-Methoxyphenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-butanamide | 2088842-68-4 | |
| para-Hydroxy‐butyrylfentanyl [27] | N-(4-hydroxyphenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-butanamide | ||
| 4"-Fluorofentanyl | N-[1-[2-(4-Fluorophenyl)ethyl]-4-piperidinyl]-N-phenylpropanamide | 2748343-99-7 | |
| 4"-Fluoro-o-fluoro-3-methylfentanyl | N-(2-fluorophenyl)-N-[1-[2-(4-fluorophenyl)ethyl]-3-methylpiperidin-4-yl]propanamide | ||
| 4-Methyl-methoxyacetylfentanyl (4-Me-MAF) | N-(4-Methylphenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-2-methoxyacetamide | ||
| 4-Methylphenethylacetylfentanyl[28] | N-[1-[2-(4-Methylphenyl)ethyl]-4-piperidinyl]-N-phenylacetamide | 1071703-95-1 | |
| Acrylfentanyl | N-Phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]prop-2-enamide | 82003-75-6 | |
| α-Methylacetylfentanyl | N-Phenyl-N-[1-(1-phenylpropan-2-yl)-4-piperidyl]acetamide | 101860-00-8 | |
| α-Methylbutyrfentanyl | N-phenyl-N-[1-(1-phenylpropan-2-yl)-4-piperidyl]butanamide | 244195-36-6 | |
| α-Methylfentanyl (AMF) | N-phenyl-N-[1-(1-phenylpropan-2-yl)-4-piperidyl]propanamide | 79704-88-4 | |
| α-Methylthiofentanyl | N-Phenyl-N-[1-(1-thiophen-2-ylpropan-2-yl)-4-piperidyl]propanamide | 103963-66-2 | |
| α-Methyl-β-hydroxyfentanyl[29] | N-[1-(1-hydroxy-1-phenylpropan-2-yl)piperidin-4-yl]-N-phenylpropanamide | ||
| Acetylfentanyl | N-(1-Phenethylpiperidin-4-yl)-N-phenylacetamide | 3258-84-2 | |
| Alfentanyl | N-{1-[2-(4-ethyl-5-oxo-4,5-dihydro-1H-1,2,3,4-tetrazol-1-yl)ethyl]-4-(methoxymethyl)piperidin-4-yl}-N-phenylpropanamide | 71195-58-9 | |
| Benzodioxolefentanyl[30] | N-Phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]-2H-1,3-benzodioxole-5-carboxamide | 2306823-01-6 | |
| Benzoylfentanyl | N-(1-Phenethylpiperidin-4-yl)-N-phenylbenzamide | 2309383-15-9 | |
| Benzylfentanyl | N-(1-Benzylpiperidin-4-yl)-N-phenylpropanamide | 1474-02-8 | |
| BDBM50223545 (Berger Fentanyl) | 1-[2-(2-phenylethyl)-3,4,4a,9b-tetrahydro-1H-pyrido[4,3-b]indol-5-yl]propan-1-one | ||
| β-Hydroxyfentanyl | N-[1-(2-Hydroxy-2-phenylethyl)piperidin-4-yl]-N-phenylpropanamide | 78995-10-5 | |
| β-Hydroxythiofentanyl | N-{1-[2-Hydroxy-2-(thiophen-2-yl)ethyl]piperidin-4-yl}-N-phenylpropanamide | 1474-34-6 | |
| β-Hydroxy-4-methylfentanyl | N-[1-(2-hydroxy-2-phenylethyl)-4-methylpiperidin-4-yl]-N-phenylpropanamide | ||
| β-Methylfentanyl | N-Phenyl-N-[1-(2-phenylpropyl)piperidin-4-yl]propanamide | 79146-56-8 | |
| Brorphine[33] | 1-{1-[1-(4-bromophenyl)ethyl]piperidin-4-yl}-1,3-dihydro-2H-benzimidazol-2-one | 2244737-98-0 | |
| Butyrfentanyl (Bu-F, BUF) | N-(1-(2-Phenylethyl)-4-piperidinyl)-N-phenylbutyramide | 1169-70-6 | |
| Brifentanyl | N-[(3R,4S)-1-[2-(4-Ethyl-5-oxotetrazol-1-yl)ethyl] -3-methylpiperidin-4-yl]-N-(2-fluorophenyl)-2-methoxyacetamide | 101345-71-5 | |
| Carfentanyl | Methyl 1-(2-phenylethyl)-4-[phenyl(propanoyl)amino]piperidine-4-carboxylate | 59708-52-0 | |
| Benzylcarfentanil | Methyl 1-benzyl-4-[phenyl(propanoyl)amino]piperidine-4-carboxylate | 61085-72-1 | |
| Ethylcarfentanil | Ethyl 1-(2-phenylethyl)-4-[phenyl(propanoyl)amino]piperidine-4-carboxylate | ||
| Crotonylfentanyl | N-Phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]-2-butenamide | 760930-59-4 | |
| Cyclopentylfentanyl | N-phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]cyclopentanecarboxamide | 2088918-01-6 | |
| Cyclobutylfentanyl [34] | N-[1-(2-Phenylethyl)piperidin-4-yl]-N-phenylcyclobutanecarboxamide | 2306827-55-2 | |
| Cyclopropylfentanyl | N-[1-(2-Phenylethyl)piperidin-4-yl]-N-phenylcyclopropanecarboxamide | 1169-68-2 | |
| EAZ-91-05, Psicofentanil | (quinuclidin-3-yl) 4-[phenyl(propanoyl)amino]-1-[2-(indol-3-yl)ethyl]piperidine-4-carboxylate | ||
| Ethoxyacetylfentanyl | 2-ethoxy-N-(1-phenethylpiperidin-4-yl)-N-phenylacetamide | ||
| Isobutyrylfentanyl | 2-methyl-N-phenyl-N-[1-(1-phenylpropan-2-yl)piperidin-4-yl]propanamide | 119618-70-1 | |
| Isofentanyl | N-(1-Benzyl-3-methylpiperidin-4-yl)-N-phenylpropanamide | 79278-40-3 | |
| Homofentanyl (N-phenylpropylnorfentanyl) [35] | N-phenyl-N-[1-(3-phenylpropyl)piperidin-4-yl]propanamide | 59708-54-2 | |
| R-4173[36] | N-phenyl-N-{1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl}propanamide | 2413-90-3 | |
| trans-phenylcyclopropyl-norfentanyl | 1-(trans-2-Phenylcyclopropyl)-4-(N-propionylanilino)piperidine. | 102504-49-4 | |
| Fentanyl azepane | N-phenyl-N-[1-(2-phenylethyl)azepan-4-yl]propanamide | ||
| Fentanyl carbamate | ethyl N-phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]carbamate | 1465-20-9 | |
| Pyridylfentany[37] | N-phenyl-N-[1-(2-pyridin-4-ylethyl)piperidin-4-yl]propanamide | 1443-41-0 | |
| Furanylbenzylfentanyl | N-Phenyl-N-(1-benzylpiperidin-4-yl)furan-2-carboxamide | 497240-21-8 | |
| Furanylfentanyl (Fu-F, FUF) | N-Phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]furan-2-carboxamide | 101345-66-8 | |
| Furanylethylfentanyl (FUEF) | N-[1-[2-(2-furanyl)ethyl]-4-piperidinyl]-N-phenyl-propanamide | 802544-02-1 | |
| 2-Benzofuranylethyl-alpha-methylfentanyl | N-(1-[1-(1-Benzofuran-2-yl)-2-propanyl]-4-piperidinyl)-N-phenylpropanamide | ||
| Fentanyl 4-methylene analogue (WO 2007/093603) | N-phenyl-N-{[1-(2-phenylethyl)piperidin-4-yl]methyl}propanamide | 947139-57-3 | |
| Indolylethylfentanyl | N-(1-[2-(1H-indol-3-yl)ethyl]-4-piperidinyl)-N-phenyl-propanamide | 58399-46-5 | |
| IQMF-4[38] | N-[1-(2-Phenylethyl)piperidin-4-yl]-N-(1-phenylpyrazol-3-yl)prop-2-enamide | 497100-48-8 | |
| Lofentanyl | methyl (3S,4R)-3-methyl-1-(2-phenylethyl)-4-[phenyl(propionyl)amino]piperidine-4-carboxylate | 61380-40-3 | |
| N-Methylnorcarfentanyl | methyl 1-methyl-4-(N-phenylpropanamido)piperidine-4-carboxylate | 59708-50-8 | |
| Methoxyacetylfentanyl (MAF) | 2-Methoxy-N-(1-phenethylpiperidin-4-yl)-N-phenylacetamide | 101345-67-9 | |
| meta-fluorofentanyl | N-(3-Fluorophenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-propanamide | 90736-22-4 | |
| Meta-fluoro-methoxyacetylfentanyl | 2-Methoxy-N-(1-phenethylpiperidin-4-yl)-N-(3-fluorophenyl)acetamide | 2306825-32-9 | |
| 4-(Methylthiazolyl)-pyrazolylethylfentanyl[39] | 1-[2-(1H-pyrazol-1-yl)ethyl]-4-(4-methylthiazol-2-yl)-4-(N-phenylpropionamido)piperidine | 120070-51-1 | |
| Mirfentanyl | N-[1-(2-Phenylethyl)piperidin-4-yl]-N-pyrazin-2-yl-2-furamide | 117523-47-4 | |
| MP102 | N-cycloheptyl-1-(2-phenylethyl)-4-(N-propanoylanilino)piperidine-4-carboxamide | ||
| MP135[40] | N-[4-{[2-(3-fluorophenyl)ethyl]carboxamido}-1-(2-phenylethyl)piperidin-4-yl]-N-benzylpropanamide | 2677687-49-7 | |
| N-(MDA)-fentanyl[41] | N-(1-(1-(benzo[d][1,3]dioxol-5-yl)propan-2-yl)piperidin-4-yl)-N-phenylpropionamide | ||
| N-(2C-B)-fentanyl | N-(1-(2-(2,5-dimethoxy-4-bromophenyl)ethyl)piperidin-4-yl)-N-phenylpropionamide | ||
| Ocfentanyl | N-(2-Fluorophenyl)-2-methoxy-N-[1-(2-phenylethyl)piperidin-4-yl]acetamide | 101343-69-5 | |
| Ohmefentanyl | N-[1-(2-hydroxy-2-phenylethyl)-3-methylpiperidin-4-yl]-N-phenylpropanamide | 78995-14-9 | |
| Ohmecarfentanil | methyl 1-(2-hydroxy-2-phenylethyl)-3-methyl-4-(N-propanoylanilino)piperidine-4-carboxylate | ||
| 4"-Fluoroohmefentanyl | methyl 1-[2-hydroxy-2-(4-fluorophenyl)ethyl]-3-methyl-4-(N-propanoylanilino)piperidine-4-carboxylate | ||
| 4"-Fluoro-ohlofentanyl | methyl 1‐[2‐(4‐fluorophenyl)‐2‐hydroxyethyl]‐3‐methyl‐4‐(N‐phenylpropanamido)piperidine‐4‐carboxylate | ||
| Orthofluorofentanyl | N-(2-Fluorophenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-propanamide | 910616-29-4 | |
| p-Bromofentanyl | N-(4-bromophenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-propanamide | 117994-23-7 | |
| p-Nitrofentanyl [42] | N-(4-Nitrophenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-propanamide | ||
| Parafluoroisobutyrylbenzylfentanyl | N-[(1-benzylpiperidin-4-yl)methyl]-N-(4-fluorophenyl)-2-methylpropanamide | ||
| 4-Fluorocyclopropylbenzylfentanyl | N-(1-benzylpiperidin-4-yl)-N-(4-fluorophenyl)cyclopropanecarboxamide | 2344231-47-4 | |
| Pivaloylfentanyl | 2,2-dimethyl-N-phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]propanamide | ||
| Pyrrole-fentanyl [43] | N-[1-(2-Phenylethyl)piperidin-4-yl]-N-pyrrol-1-ylpropanamide | ||
| R-30490 | N-[4-(Methoxymethyl)-1-(2-phenylethyl)piperidin-4-yl]-N-phenylpropanamide | 60618-49-7 | |
| Remifentanyl | methyl 1-(3-methoxy-3-oxopropyl)-4-(N-phenylpropanamido)piperidine-4-carboxylate | 132875-61-7 | |
| Remifentanil bis ethyl ester | ethyl 1-(3-ethoxy-3-oxopropyl)-4-(N-phenylpropanamido)piperidine-4-carboxylate | ||
| RR49 [44] | N-{1-[2-Fluoro-2-(2-fluorophenyl)ethyl]piperidin-4-yl}-N-phenylpropanamide | 2376328-79-7 | |
| SR-16412 [45] | 1-(1-benzylpiperidin-4-yl)-3H-indol-2-one | 16223-24-8 | |
| Secofentanyl[46] | N-phenyl-N-{4-[methyl(2-phenylethyl)amino]butan-2-yl}propanamide | 253342-66-4 | |
| Senecioylfentanyl [47] | N-phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]-3-methylbut-2-enamide | 2630378-28-6 | |
| Sufentanil | N-[4-(Methoxymethyl)-1-(2-thiofuran-2-ylethyl)-4-piperidyl]-N-phenylpropanamide | 56030-54-7 | |
| Tetrahydrofuranylfentanyl | N-Phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]oxolane-2-carboxamide | 2142571-01-3 | |
| Tetramethylcyclopropylfentanyl[48] | 2,2,3,3-Tetramethyl-N-(1-phenethylpiperidin-4-yl)-N-phenylcyclopropane-1-carboxamide | 2309383-11-5 | |
| Tetramethylfentanyl | N-(2,2,6,6-tetramethyl-1-phenethyl-4-piperidyl)-N-phenyl-propanamide | ||
| Thenylfentanyl | N-phenyl-N-{[1-(thiophen-2-ylmethyl)piperidin-4-yl]methyl}propanamide | 117332-93-1 | |
| Thiafentanil | methyl 4-(N-(2-methoxyacetyl)anilino)-1-(2-thiophen-2-ylethyl)piperidine-4-carboxylate | 101345-60-2 | |
| Thiofentanyl | N-phenyl-N-{1-[2-(2-thienyl)ethyl]piperidin-4-yl}propanamide | 1165-22-6 | |
| Thiophenylfentanyl (Thiofuranylfentanyl)[49] | N-Phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]thiophene-2-carboxamide | 2306823-38-9 | |
| Trefentanyl | N-{1-[2-(4-ethyl-5-oxo-4,5-dihydro-1H-tetrazol-1-yl)ethyl]-4-phenylpiperidin-4-yl}-N-(2-fluorophenyl)propanamide | 120656-93-1 | |
| Trifluorofentanyl [50] | N-(3,4-difluorophenyl)-N-[1-(2-[4-fluorophenyl]ethyl)-4-piperidinyl]-propanamide | ||
| Tropafentanyl (Fentanyl tropane) | N-phenyl-N-[8-(2-phenylethyl)-8-azabicyclo[3.2.1]octan-3-yl]propanamide | ||
| 3,4-dichloro-4''-methoxyfentanyl | N-(3,4-dichlorophenyl)-N-[1-(2-[4-methoxyphenyl]ethyl)-4-piperidinyl]-propanamide | 1161705-29-8 | |
| Ureafentanyl | N,N-dimethyl-N'-phenyl-N'-[1-(2-phenylethyl)-4-piperidinyl]-urea | 1443-50-1 | |
| Valerylfentanyl (VF) | N-(1-(2-Phenylethyl)-4-piperidinyl)-N-phenylpentylamide | 122882-90-0 |
Analogue controls
editSeveral jurisdictions have implemented analogue law controls of fentanyl analogues in an attempt to pre-emptively ban novel derivatives before they appear on the market. One representative example is the New Zealand provisions enacted in 1988 in response to the first wave of fentanyl derivatives. This bans a set of structures as follows;
"Fentanyl analogues, in which the N-[1-(2-phenethyl)-4-piperidyl]aniline nucleus has additional radicals, either alone or in combination, attached as follows:
(a) an acetyl, propionyl, butenoyl or butanoyl radical, attached to the aniline nitrogen atom:
(b) 1 or more alkyl radicals, with up to 10 carbon atoms in total, attached to the ethyl moiety:
(c) any combination of up to 5 alkyl radicals and/or alkoxy radicals (each with up to 6 carbon atoms, including cyclic radicals) and/or halogen radicals, attached to each of the benzene rings."[51]
A more recent and somewhat broader example was introduced into US Federal legislation in 2018,[52] covering the following structures;
"...fentanyl-related substances includes any substance not otherwise controlled in any schedule...that is structurally related to fentanyl by one or more of the following modifications:
- Replacement of the phenyl portion of the phenethyl group by any monocycle, whether or not further substituted in or on the monocycle;
- substitution in or on the phenethyl group with alkyl, alkenyl, alkoxyl, hydroxyl, halo, haloalkyl, amino or nitro groups;
- substitution in or on the piperidine ring with alkyl, alkenyl, alkoxyl, ester, ether, hydroxyl, halo, haloalkyl, amino or nitro groups;
- replacement of the aniline ring with any aromatic monocycle whether or not further substituted in or on the aromatic monocycle; and/or
- replacement of the N-propionyl group by another acyl group."[53]
Organic chemistry
editBecause there are so many analogues of fentanyl, the naming for them tends to follow classical or IUPAC nomenclature conventions. This section is written to help illustrate the basic ring structure of fentanyl and what popular analogues reference on the carbon skeleton, helping a chemist working with fentanyl analogues quickly and consistently navigate the nomenclature system.
- Part I
The synthesis of fentanyl and its analogues are illustrated in these skeletal diagrams. The synthesis of fentalogs is done by reacting the ring structure as a base, 4-ANPP also called 4-anilino-N-phenethylpiperidine and despropionylfentanyl. 4-ANPP acts as a base because of its two amine nitrogens, the secondary amine acts as a base to react with an organic acid which condenses into an amide. Depending on the organic acid used in the amide condensation, different analogues of fentanyl will be produced. To help align this phenomenon in the eyes of chemists, we have numerated the precursor 4-ANPP with a [0.], and then the reactions synthesizing fentanyl, acetylfentanyl, butyrylfentanyl, and benzoylfentanyl with a [1.], [2.], [3.], and [4.] respectively. To further aid in the chemical pedagogy we have aligned each number with a reaction number -> precursor -> product system that follows the following scheme: EXAMPLE: reaction number -> precursor -> product [compound name] 1 -> α -> a [fentanyl] 2 -> β -> b [acetylfentanyl] 3 -> γ -> c [butyrylfentanyl] 4 -> δ -> d [benzoylfentanyl]
Using the above scheme, a chemist can quickly extrapolate the reactions necessary for other fentanyl analogues with more complex organic acids, such as cyclopropryl fentanyl or cyclopentyl fentanyl, or any other fentanyl analogue derived from a reaction of 4-ANPP with a novel organic acid.
- Part II
The modifications covered in this diagram have to do with carbon skeleton modifications of the original fentanyl molecular structure. These are organized into methyl acetate additions, which are most known for the fentanyl -> carfentanil conversion. Many analogues of great potency, such as ohmfentanyl and lofentanyl possess methyl acetate groups added to the 4-carbon (of the piperidine ring, in the para- position relative to the annular nitrogen). The methyl acetate is added here from the α-carbon of the acetate moiety as it is with ohm- and lofentanyl. The 4-carbon is not a chiral center in carfetnanil because of a lack of piperidinyl substituents, but this same carbon is a chiral center in both ohm- and lofentanyl because both of those analogues have piperidinyl substituents.
The second group are organized into methyl additions, which are known for the fentanyl analogues such as α-methylfentanyl and cis-3-methylfentanyl. These analogues can possess a wide variety of modified pharmacological properties, including increased and decreased potency (receptor binding efficiency), increased or decreased half-life (metabolic binding efficiency) or other side effects on human physiology. Other substituents such as hydroxy, chloro, fluoro, and a wide variety of alkyl groups, are also substituted in place of these methylations to produce psychoactive analogues of fentanyl, but because they often use the same skeletal naming conventions as the simple methyl analogues, we did not reproduce them all in the image here.
- Part III
The modifications described here cover alterations to the ring structure donated by the fentanyl precursor 4-ANPP. Although another series covered substitutions of hydrogen atoms on the original carbon ring structure, sometimes called functional group additions, this series focuses on the three main modifications to the phenethyl moiety. The first modifications is the removal of the phenthyl moeity from the piperidinyl nitrogen, depicted here as hydrolysis yielding phenethanol. This changes the parent skeletal name to norfentanyl.
The second and third modifications are the removal and addition of a methylene moiety internally in the ethyl chain within the greater phenethyl moiety. The removal of a methylene, which shortens the chain by one carbon length, creates the benzylfentanyl structure. The addition of a methylene, which lengthens the chain by one length, creates homofentanyl.
- Part IV
The modifications covered in this diagram have to do with stereochemistry and the assignment of unique Cahn-Ingold-Prelog R/S assignments to complex analogues of fentanyl. The stereochemistry of fentanyl analgoues can seem at first counter-intuitive, because of the complex and unique nature of the reasoning organic chemists must develop to internalize complex three dimensional geometries such as those needed to comprehend stereochemistry. Thankfully these images follow a simple procedure for organizing a potential analogue of fentanyl into the total number of unique stereoisomers, the number of true stereocenters on the molecule, and the number of Cahn-Ingold-Prelog R/S assignments that are appropriate for that analogue.
The procedure used in the analysis of stereochemistry in these series of images is the following:
- First the image is drawn out.
- Second asterisks are placed by potential stereocenters as indicators (*).
- Third the pairs of each stereocenters R and S orientations are combined through all possible permutations of stereocenters.
- Fourth the permutations are checked for super-imposability, indicating that they are varying around what is not a true stereocenter and therefore the permutations are truly the self-same stereoisomer.
This procedure will be used when making an assessment of the number of valid stereoisomers that an analogue of fentanyl will be predicted to have. Because the analogues of fentanyl are so large, and the moieities (sub-regions) of the molecule that are relevant to its chirality are so small, that we often reproduce the fentanyl analogue as a smaller, simpler molecule with the same number of, and dynamics between its, stereocenters. This saves significant space allowing us not to reproduce redundant material that consumes a lot of space on the image.
The first case studied here is fentanyl itself, or propionyl-4-anilino-N-phenethylpiperidine, the most well known of the fentanyl analogues and the eponymous molecule (namesake) for the whole chemical categorey. The only identified stereocenter is found at the 4-carbon, opposite the piperidine nitrogen but adjacent to the aniline nitrogen. This seems to be a stereocenter because of the apparent 4 unique substituents. We transfer this stereocenter to the equivalent molecule cyclohexanol, with an analogous apparent stereocenter. Once we draw out the potential stereoisomers, we see that the two structures are super-imposable in three dimensions, and therefore are the self-same molecule. For this reason fentanyl does not have R/S assignments.
The second case studied here is of 3-methylfentanyl. There are two potential stereocenters, at the 4-carbon and also at the 3-carbon, where there is additionally a methyl group. Now, we mark both 3 and 4 carbon as potential stereocenters with an asterisk (*), and see how many potential stereoisomers we can eliminate. We see that of the four permutations of stereoisomers, none are super-imposable in three dimensions, meaning each is a unique stereoisomer and that both potential stereocenters were true. This gives 4 potential R/S assignments, 1R3S, 1R3R, 1S3S, 1S3R. What is particularly interesting and quite a contrast from the previous example of cyclohexanol, is the stereocenter at C-4 is indeed a true stereocenter here, whereas in the previous example of cyclohexanol as an analogy to fentanyl, the C-4 was not a true stereocenter. This changed because the modification of the C-3 carbon without an equivalent change on the C-2 carbon created an imbalance between two formerly identical substituents, creating a novel stereocenter where there was not one previously. This is why it is so important to follow the 4 steps in the above procedure every time, as "inherited procedural wisdom" may hold an organic chemist back in finding the true or correct answer.
- Part V
The third case studied here is alpha-methylfentanyl. This group contains a substitution similar to the 3-methylfentanyl that was examined in the previous example on the previous image in this series (Fentanyl Synthesis p4.png), but it is on the phenethyl chain as opposed to being implanted on the piperidine ring. We then mark the two stereocenters, one on the C-4 carbon just as on fentanyl itself, and the other on the C-α (alpha carbon). To analogize these stereocenters we chose N-(1-hydroxyethyl)-4-hydroxypiperidine. When we draw out all potential stereoisomers, we see that the C-4 stereocenter is super-imposable, eliminating it as a true stereocenter. This leaves only 2 R/S assignments that follow the orientation of the stereocenter at the C-α (alpha carbon) position for the real α-methylfentanyl. These are labeled 7S and 7R, a reflection of the stereocenter in the piperidine derivative we used being at C-7 position. It is interesting that the C-4 stereocenter, which has no chiral activity in fentanyl (1st example), activiates it in 3-methylfentanyl with an on-the-ring addition, and loses it again in α-methylfentanyl with an off-the-ring addition.
The fourth case studied here is ohmfentanyl. Ohmfentanyl has 3 potential stereocenters, which are best analogized by another piperidine derivative: N-(1-hydroxyethyl)-3-methyl-4-hydroxypiperidine. This uses a similar structure to analogize the three potential stereocenters in ohmfentanyl, namely the 4-C, the 3-C, and the β-C (beta carbon). These three stereocenters are analogized in the C-4, C-3, and C-7 respectively. When we draw all potential stereoisomers out we see that they are not super-imposable in any combination of pairing, and therefore we have 8 unique stereoisomers and 3 true stereocenters. This yields 8 unique R/S assignment combinations which are outlined as captions under the stereoisomers of the analogous molecule.
These fundamentals are typically enough to help a chemists navigate the world of fentanyl analogues proficiently. Other substituents and substitutions generally follow the naming conventions outlined in this section. However, the presence of three six-membered rings which can each be independently substituted can easily lead to confusion, especially with the inconsistent use of prime notation.
For instance, 4-methylfentanyl, 4'-methylfentanyl and 4"-methylfentanyl are all known compounds, as are 3-methylthio-fentanyl and 3-methyl-thiofentanyl, all of which have varying potencies and pharmacokinetics.
Confusion between different positional isomers is especially significant in the case of fentanyls because of the huge variation in potency between different members of the class.[54] The weakest compounds such as benzylfentanyl are around the same potency as codeine (i.e. approximately 1/10th the potency of morphine), while the strongest compounds such as carfentanil and ohmefentanil can be over 10,000x more potent than morphine, meaning there is a 100,000-fold variation in potency between the strongest and weakest fentanyl derivatives. This means that two positional isomers with the same molecular weight, which may be difficult to tell apart without detailed chemical analysis, may be hundreds or even thousands of times different in pharmacological potency. Also the wide variety of substitutions that have been used on the basic fentanyl structure, each of which can either reduce or increase the potency, can be unpredictable when used in combination, so it may be impossible to estimate the likely potency of newly discovered analogues until pharmacological testing has been carried out.
See also
editReferences
edit- ^ Hassanien SH, Bassman JR, Perrien Naccarato CM, Twarozynski JJ, Traynor JR, Iula DM, Anand JP (August 2020). "In vitro pharmacology of fentanyl analogs at the human mu opioid receptor and their spectroscopic analysis". Drug Testing and Analysis. 12 (8): 1212–1221. doi:10.1002/dta.2822. PMC 8034271. PMID 32415719.
- ^ Vaughan SR, Fulton AC, DeGreeff LE. Comparative analysis of vapor profiles of fentalogs and illicit fentanyl. Anal Bioanal Chem 413, 7055–7062 (2021). doi:10.1007/s00216-021-03670-4
- ^ Patel JC, Parveen S (January 2022). "In Vitro and In Vivo Analysis of Fentanyl and Fentalog Metabolites using Hyphenated Chromatographic Techniques: A Review". Chemical Research in Toxicology. 35 (1): 30–42. doi:10.1021/acs.chemrestox.1c00225. PMID 34957817. S2CID 245500557.
- ^ Mounteney J, Giraudon I, Denissov G, Griffiths P (July 2015). "Fentanyls: Are we missing the signs? Highly potent and on the rise in Europe". The International Journal on Drug Policy. 26 (7): 626–31. doi:10.1016/j.drugpo.2015.04.003. PMID 25976511.
- ^ Zawilska JB (2017). "An Expanding World of Novel Psychoactive Substances: Opioids". Frontiers in Psychiatry. 8: 110. doi:10.3389/fpsyt.2017.00110. PMC 5492455. PMID 28713291.
- ^ "Recommended Methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens" (PDF). United Nations Office on Drugs and Crime. November 2017.
- ^ Vardanyan RS, Hruby VJ (March 2014). "Fentanyl-related compounds and derivatives: current status and future prospects for pharmaceutical applications". Future Medicinal Chemistry. 6 (4): 385–412. doi:10.4155/fmc.13.215. PMC 4137794. PMID 24635521.
- ^ Armenian P, Vo KT, Barr-Walker J, Lynch KL (May 2018). "Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review". Neuropharmacology. 134 (Pt A): 121–132. doi:10.1016/j.neuropharm.2017.10.016. PMID 29042317. S2CID 21404877.
- ^ "Fentanyl-related substances with no known legitimate uses" (PDF). International Narcotics Control Board. 24 June 2018.
- ^ Choińska MK, Šestáková I, Hrdlička V, Skopalová J, Langmaier J, Maier V, Navrátil T (January 2022). "Electroanalysis of Fentanyl and Its New Analogs: A Review". Biosensors. 12 (1): 26. doi:10.3390/bios12010026. PMC 8774265. PMID 35049654.
- ^ Bagley JR, Kudzma LV, Lalinde NL, Colapret JA, Huang BS, Lin BS, et al. (July 1991). "Evolution of the 4-anilidopiperidine class of opioid analgesics". Medicinal Research Reviews. 11 (4): 403–36. doi:10.1002/med.2610110404. PMID 1875771. S2CID 33000913.
- ^ Skulska A, Kała M, Parczewski A (2004). "Fentanyl and its analogs in the forensic laboratory. Medical and analytical problems" (PDF). Probl Forensic Sci. 59: 127–42.
- ^ Misailidi N, Papoutsis I, Nikolaou P, Dona A, Spiliopoulou C, Athanaselis S (January 2018). "Fentanyls continue to replace heroin in the drug arena: the cases of ocfentanil and carfentanil". Forensic Toxicology. 36 (1): 12–32. doi:10.1007/s11419-017-0379-4. PMC 5754389. PMID 29367860.
- ^ Wilde M, Pichini S, Pacifici R, Tagliabracci A, Busardò FP, Auwärter V, Solimini R (2019). "Metabolic Pathways and Potencies of New Fentanyl Analogs". Frontiers in Pharmacology. 10: 238. doi:10.3389/fphar.2019.00238. PMC 6461066. PMID 31024296.
- ^ Sofalvi S, Lavins ES, Brooker IT, Kaspar CK, Kucmanic J, Mazzola CD, et al. (October 2019). "Unique Structural/Stereo-Isomer and Isobar Analysis of Novel Fentanyl Analogues in Postmortem and DUID Whole Blood by UHPLC-MS-MS". Journal of Analytical Toxicology. 43 (9): 673–687. doi:10.1093/jat/bkz056. PMID 31504606.
- ^ Varshneya NB, Walentiny DM, Moisa LT, Walker TD, Akinfiresoye LR, Beardsley PM (June 2019). "Opioid-like antinociceptive and locomotor effects of emerging fentanyl-related substances". Neuropharmacology. 151: 171–179. doi:10.1016/j.neuropharm.2019.03.023. PMC 8992608. PMID 30904478.
- ^ Varshneya NB, Walentiny DM, Moisa LT, Walker TD, Akinfiresoye LR, Beardsley PM (September 2021). "Fentanyl-related substances elicit antinociception and hyperlocomotion in mice via opioid receptors". Pharmacology, Biochemistry, and Behavior. 208: 173242. doi:10.1016/j.pbb.2021.173242. PMC 9044821. PMID 34302853.
- ^ Varshneya NB, Hassanien SH, Holt MC, Stevens DL, Layle NK, Bassman JR, et al. (January 2022). "Respiratory depressant effects of fentanyl analogs are opioid receptor-mediated". Biochemical Pharmacology. 195: 114805. doi:10.1016/j.bcp.2021.114805. PMC 9022371. PMID 34673011.
- ^ Varshneya NB, Walentiny DM, Stevens DL, Walker TD, Akinfiresoye LR, Beardsley PM (January 2023). "Structurally diverse fentanyl analogs yield differential locomotor activities in mice". Pharmacology, Biochemistry, and Behavior. 222: 173496. doi:10.1016/j.pbb.2022.173496. PMC 9845183. PMID 36435268.
- ^ Varshneya NB, Hassanien SH, Holt MC, Stevens DL, Layle NK, Bassman JR, et al. (May 2023). "Fentanyl analog structure-activity relationships demonstrate determinants of diverging potencies for antinociception and respiratory depression". Pharmacology, Biochemistry, and Behavior. 226: 173572. doi:10.1016/j.pbb.2023.173572. PMC 10326888. PMID 37236405.
- ^ Drug Enforcement Administration (February 2018). "Schedules of Controlled Substances: Temporary Placement of Fentanyl-Related Substances in Schedule I. Temporary amendment; temporary scheduling order". Federal Register. 83 (25). Department of Justice: 5188–92. PMID 29932611.
- ^ "Fentanyl-Related Substances" (PDF). Drug Enforcement Administration, Diversion Control Division, Drug & Chemical Evaluation Section. Department of Justice. January 2023.
- ^ Lalinde N, Moliterni J, Wright D, Spencer HK, Ossipov MH, Spaulding TC, Rudo FG (October 1990). "Synthesis and pharmacological evaluation of a series of new 3-methyl-1,4-disubstituted-piperidine analgesics". Journal of Medicinal Chemistry. 33 (10): 2876–82. doi:10.1021/jm00172a032. PMID 2170652.
- ^ Rautio T, Vangerven D, Dahlén J, Watanabe S, Kronstrand R, Vikingsson S, et al. (February 2023). "In vitro metabolite identification of acetylbenzylfentanyl, benzoylbenzylfentanyl, 3-fluoro-methoxyacetylfentanyl, and 3-phenylpropanoylfentanyl using LC-QTOF-HRMS together with synthesized references". Drug Testing and Analysis. 15 (7): 711–729. doi:10.1002/dta.3454. PMID 36756728.
- ^ Le AD, Alzghari SK (October 2019). "Systematic review of the clinical consequences of butyrfentanyl and corresponding analogues". Interdisciplinary Toxicology. 12 (2): 83–88. doi:10.2478/intox-2019-0009. PMC 7071838. PMID 32206028.
- ^ Vincenti F, Montesano C, Di Ottavio F, Gregori A, Compagnone D, Sergi M, Dorrestein P (2020). "Molecular Networking: A Useful Tool for the Identification of New Psychoactive Substances in Seizures by LC-HRMS". Frontiers in Chemistry. 8: 572952. doi:10.3389/fchem.2020.572952. PMC 7723841. PMID 33324608.
- ^ Oldenhof S, Ten Pierick A, Bruinsma J, Eustace S, Hulshof J, van den Berg J, Hoitink M (January 2020). "Identification of a novel fentanyl analog: p-Hydroxy-butyrylfentanyl". Drug Testing and Analysis. 12 (1): 152–155. doi:10.1002/dta.2695. PMID 31518047. S2CID 202569865.
- ^ Moore JM, Allen AC, Cooper DA, Carr SM (July 1986). "Determination of fentanyl and related compounds by capillary gas chromatography with electron capture detection". Analytical Chemistry. 58 (8): 1656–60. doi:10.1021/ac00121a013. PMID 3752503.
- ^ Barceloux DG (2012). Medical Toxicology of Drug Abuse: Synthesized Chemicals and Psychoactive Plants. Wiley. ISBN 978-0471727606.
- ^ Varshneya NB, Walentiny DM, Moisa LT, Walker TD, Akinfiresoye LR, Beardsley PM (June 2019). "Opioid-like antinociceptive and locomotor effects of emerging fentanyl-related substances". Neuropharmacology. 151: 171–179. doi:10.1016/j.neuropharm.2019.03.023. PMC 8992608. PMID 30904478. S2CID 84182661.
- ^ Berger, J. G., Davidson, F., Langford, G. E. (April 1977). "Synthesis of some conformationally restricted analogs of fentanyl". Journal of Medicinal Chemistry. 20 (4): 600–602. doi:10.1021/jm00214a035. ISSN 0022-2623. PMID 850249. Retrieved November 18, 2022.
- ^ Lobbezoo MW, Soudijn W, van Wijngaarden I (July 1981). "Opiate receptor interaction of compounds derived from or structurally related to fentanyl". Journal of Medicinal Chemistry. 24 (7): 777–782. doi:10.1021/jm00139a003. PMID 6268786.
- ^ Kennedy NM, Schmid CL, Ross NC, Lovell KM, Yue Z, Chen YT, et al. (October 2018). "Optimization of a Series of Mu Opioid Receptor (MOR) Agonists with High G Protein Signaling Bias". Journal of Medicinal Chemistry. 61 (19): 8895–8907. doi:10.1021/acs.jmedchem.8b01136. PMC 6386185. PMID 30199635.
- ^ Elbardisy HM, Foster CW, Cumba L, Antonides LH, Gilbert N, Schofield CJ, et al. (2019). "Analytical determination of heroin, fentanyl and fentalogues using high-performance liquid chromatography with diode array and amperometric detection". Analytical Methods. 11 (8): 1053–63. doi:10.1039/C9AY00009G.
- ^ Cooper D, Jacob M, Allen A (April 1986). "Identification of fentanyl derivatives". Journal of Forensic Sciences. 31 (2): 511–28. doi:10.1520/JFS12283J. PMID 3711827.
- ^ US granted 3161637, Janssen PA, "1-(gamma-aroyl-propyl)-4-(nu-arylcarbonyl amino) piperidines and related compounds", issued 15 December 1964, assigned to Janssen Research Labs
- ^ Robertson TB, Antonides LH, Gilbert N, Benjamin SL, Langley SK, Munro LJ, Sutcliffe OB, Mewis RE (December 2019). "Hyperpolarization of Pyridyl Fentalogues by Signal Amplification By Reversible Exchange (SABRE)". ChemistryOpen. 8 (12): 1375–1382. doi:10.1002/open.201900273. PMC 6892445. PMID 31844604.
- ^ Goicoechea C, Sánchez E, Cano C, Jagerovic N, Martín MI (October 2008). "Analgesic activity and pharmacological characterization of N-[1-phenylpyrazol-3-yl]-N-[1-(2-phenethyl)-4-piperidyl] propenamide, a new opioid agonist acting peripherally". European Journal of Pharmacology. 595 (1–3): 22–9. doi:10.1016/j.ejphar.2008.07.052. PMID 18706410.
- ^ Kudzma LV, Severnak SA, Benvenga MJ, Ezell EF, Ossipov MH, Knight VV, et al. (December 1989). "4-Phenyl- and 4-heteroaryl-4-anilidopiperidines. A novel class of analgesic and anesthetic agents". Journal of Medicinal Chemistry. 32 (12): 2534–2542. doi:10.1021/jm00132a007. PMID 2585442.
- ^ Faouzi A, Uprety R, Gomes I, Massaly N, Keresztes AI, Le Rouzic V, Gupta A, Zhang T, Yoon HJ, Ansonoff M, Allaoa A, Pan YX, Pintar J, Morón JA, Streicher JM, Devi LA, Majumdar S (November 2020). "Synthesis and Pharmacology of a Novel μ-δ Opioid Receptor Heteromer-Selective Agonist Based on the Carfentanyl Template". Journal of Medicinal Chemistry. 63 (22): 13618–13637. doi:10.1021/acs.jmedchem.0c00901. PMC 7745089. PMID 33170687.
- ^ W De Silva I, Couch AN, Verbeck GF (December 2020). "Paper Spray Mass Spectrometry Utilized with a Synthetic Microporous Polyolefin Silica Matrix Substrate in the Rapid Detection and Identification of More than 190 Synthetic Fentanyl Analogs". Journal of the American Society for Mass Spectrometry. 32 (2): 420–428. doi:10.1021/jasms.0c00250. PMID 33296202. S2CID 228088741.
- ^ Stojanović A, Jevtic I, Krunić M, Ivanovic M (October 2022). Synthesis of para-nitro fentanyl. 8th Conference of Young Chemists of Serbia.
- ^ US 4452803, Effland RC, Klein JT, "Pyrrolylaminopiperidines.", issued 19 September 1983
- ^ Rosas R, Huang XP, Roth BL, Dockendorff C (September 2019). "β-Fluorofentanyls Are pH-Sensitive Mu Opioid Receptor Agonists". ACS Medicinal Chemistry Letters. 10 (9): 1353–1356. doi:10.1021/acsmedchemlett.9b00335. PMC 6746189. PMID 31531209.
- ^ Zaveri NT, Jiang F, Olsen CM, Deschamps JR, Parrish D, Polgar W, Toll L (June 2004). "A novel series of piperidin-4-yl-1,3-dihydroindol-2-ones as agonist and antagonist ligands at the nociceptin receptor". Journal of Medicinal Chemistry. 47 (12): 2973–6. doi:10.1021/jm034249d. PMC 3852901. PMID 15163178.
- ^ Ivanović MD, Mićović I, Vučković S, Prostran M, Todorović Z, Ivanović E, Kiricojević V, Đorđević JB, Došen-Mićović L (2004). "The synthesis and pharmacological evaluation of (+/-)-2, 3-seco-fentanyl analogues". Journal of the Serbian Chemical Society. 69 (11): 955–68. doi:10.2298/JSC0411955I.
- ^ Bergh MS, Bogen IL, Nerem E, Wohlfarth A, Wilson SR, Øiestad ÅM (2021). "Discovering the major metabolites of the three novel fentanyl analogues 3-methylcrotonylfentanyl, furanylbenzylfentanyl, and 4-fluorocyclopropylbenzylfentanyl for forensic case work". Forensic Toxicology. 39: 167–178. doi:10.1007/s11419-020-00560-9. S2CID 230110612.
- ^ Åstrand A, Vikingsson S, Jakobsen I, Björn N, Kronstrand R, Gréen H (August 2020). "Activation of the μ-opioid receptor by alicyclic fentanyls: Changes from high potency full agonists to low potency partial agonists with increasing alicyclic substructure". Drug Testing and Analysis. 13 (1): 169–174. doi:10.1002/dta.2906. PMC 7891621. PMID 32749741.
- ^ Popławska, M., Bednarek, E., Naumczuk, B. et al. Identification and structure characterization of five synthetic opioids: 3,4-methylenedioxy-U-47700, o-methyl-acetylfentanyl, 2-thiophenefentanyl, benzoylfentanyl and benzoylbenzylfentanyl. Forensic Toxicol 39, 45–58 (2021) doi:10.1007/s11419-020-00539-6
- ^ US abandoned 2011046180, Peters D, Eriksen BL, Munro G, Nielsen EØ, "N-aryl-n-piperidin-4-yl-propionamide derivatives and their use as opioid receptor ligands", assigned to Neurosearch AS
- ^ "Schedule 3 Class C Controlled Drugs; Part 7: Fentanyl analogues". New Zealand Misuse of Drugs Act 1975.
- ^ Weedn VW, Elizabeth Zaney M, McCord B, Lurie I, Baker A (July 2021). "Fentanyl-related substance scheduling as an effective drug control strategy". Journal of Forensic Sciences. 66 (4): 1186–1200. doi:10.1111/1556-4029.14712. PMC 8360110. PMID 33951192.
- ^ "Schedules of Controlled Substances: Temporary Placement of Fentanyl-Related Substances in Schedule I". United States Federal Register. 6 February 2018.
- ^ Varshneya NB, Hassanien SH, Holt MC, Stevens DL, Layle NK, Bassman JR, et al. (May 2023). "Fentanyl analog structure-activity relationships demonstrate determinants of diverging potencies for antinociception and respiratory depression". Pharmacology, Biochemistry, and Behavior. 226: 173572. doi:10.1016/j.pbb.2023.173572. PMC 10326888. PMID 37236405.
External links
edit- "Fentanyl landscape | PiHKAL · info". isomerdesign.com.