The Friedländer synthesis is a chemical reaction of 2-aminobenzaldehydes[1] with ketones to form quinoline derivatives.[2][3] It is named after German chemist Paul Friedländer (1857–1923).
| Friedländer synthesis | |
|---|---|
| Named after | Paul Friedländer |
| Reaction type | Ring forming reaction |
| Identifiers | |
| Organic Chemistry Portal | friedlaender-synthesis |
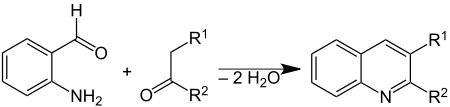
This reaction has been catalyzed by trifluoroacetic acid,[4] toluenesulfonic acid,[5] iodine,[6] and Lewis acids.[7]
Mechanism
editTwo viable reaction mechanisms exist for this reaction. In the first mechanism 2-amino substituted carbonyl compound 1 and carbonyl compound 2 react in a rate-limiting step to aldol adduct 3. This intermediate loses water in an elimination reaction to unsaturated carbonyl compound 4 and then loses water again in imine formation to quinoline 7. In the second mechanism the first step is Schiff base formation to 5 followed by Aldol reaction to 6 and elimination to 7.[11]
The Pfitzinger reaction and the Niementowski quinoline synthesis are variations of the Friedländer reaction.
See also
editReferences
edit- ^ Organic Syntheses, Coll. Vol. 3, p. 56 (1955); Vol. 28, p. 11 (1948). (Article)
- ^ Friedländer, P. (1882). "Ueber o-Amidobenzaldehyd". Chemische Berichte. 15 (2): 2572–2575. doi:10.1002/cber.188201502219.
- ^ Friedländer, P.; Gohring, C. F. (1883). "Ueber eine Darstellungsmethode im Pyridinkern substituirter Chinolinderivate". Ber. 16 (2): 1833–1839. doi:10.1002/cber.18830160265.
- ^ Shaabani, A.; Soleimani, E.; Badri, Z. (2007). "Triflouroacetic Acid as an Efficient Catalyst for the Synthesis of Quinoline". Synthetic Communications. 37 (4): 629–635. doi:10.1080/00397910601055230. S2CID 98625429.
- ^ Jia, C.-S.; Zhang, Z.; Tu, S.-J.; Wang, G.-W. (2006). "Rapid and efficient synthesis of poly-substituted quinolines assisted by p-toluene sulphonic acid under solvent-free conditions: Comparative study of microwave irradiation versus conventional heating". Org. Biomol. Chem. 4 (1): 104–110. doi:10.1039/b513721g. PMID 16358003.
- ^ Wu, J.; Xia, H.-G.; Gao, K. (2006). "Molecular iodine: A highly efficient catalyst in the synthesis of quinolines via Friedländer annulation". Org. Biomol. Chem. 4 (1): 126–129. doi:10.1039/b514635f. PMID 16358006.
- ^ Varala, R.; Enugala, R.; Adapa, S. R. (2006). "Efficient and Rapid Friedlander Synthesis of Functionalized Quinolines Catalyzed by Neodymium(III) Nitrate Hexahydrate". Synthesis. 2006 (22): 3825–3830. doi:10.1055/s-2006-950296.
- ^ Manske, R. H. (1942). "The Chemistry of Quinolines". Chem. Rev. 30: 113–144. doi:10.1021/cr60095a006.
- ^ Bergstrom, F. W. (1944). "Heterocyclic Nitrogen Compounds. Part IIA. Hexacyclic Compounds: Pyridine, Quinoline, and Isoquinoline". Chem. Rev. 35 (2): 77–277. doi:10.1021/cr60111a001.
- ^ Cheng, C.-C.; Yan, S.-J. (2004). "The Friedländer Synthesis of Quinolines". Organic Reactions. doi:10.1002/0471264180.or028.02. ISBN 0471264180.
- ^ Jose Marco-Contelles; Elena Perez-Mayoral; Abdelouahid Samadi; Marıa do Carmo Carreiras; Elena Soriano (2009). "Recent Advances in the Friedlander Reaction". Chemical Reviews. 109 (6): 2652–71. doi:10.1021/cr800482c. PMID 19361199.