Human metapneumovirus (HMPV or hMPV) is a negative-sense single-stranded RNA virus of the family Pneumoviridae[1] and is closely related to the Avian metapneumovirus (AMPV) subgroup C. It was isolated for the first time in 2001 in the Netherlands by using the RAP-PCR (RNA arbitrarily primed PCR) technique for identification of unknown viruses growing in cultured cells.[2] As of 2016, it was the second most common cause (after respiratory syncytial virus (RSV)) of acute respiratory tract illness in otherwise-healthy children under the age of 5 in a large US outpatient clinic.[3]
| Human metapneumovirus | |
|---|---|
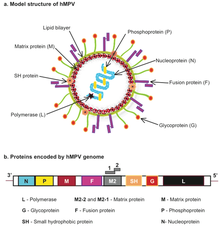
| |
| Human metapneumovirus (hMPV) structure and genome | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Negarnaviricota |
| Class: | Monjiviricetes |
| Order: | Mononegavirales |
| Family: | Pneumoviridae |
| Genus: | Metapneumovirus |
| Species: | Human metapneumovirus
|
The peak age of hospitalization for infants with HMPV occurs between 6–12 months of age, slightly older than the peak of RSV, which is around 2–3 months. The clinical features and severity of HMPV are similar to those of RSV. HMPV is also an important cause of disease in older adults.
Taxonomy
edit| Genus | Species | Virus (abbreviation) | NCBI taxonomy ID |
|---|---|---|---|
| Metapneumovirus | Avian metapneumovirus | avian metapneumovirus (AMPV) | 38525 |
| Human metapneumovirus | human metapneumovirus (HMPV) | 162145 |
Discovery and naming
editHuman metapneumovirus was first discovered in 2001 in the Netherlands by Bernadette G. van den Hoogen and her colleagues.[5][6][7][8] hMPV was first detected in the respiratory secretions of 28 young children in the Netherlands and had initially stood out from other common respiratory viruses because the testing methods van den Hoogen et al. had tried using (immunological assays using virus-specific antibodies and PCR-based methods using virus genome-specific primers) were only able to test for known respiratory viruses and, therefore, were unable to identify the novel virus.[5] It was not until researchers began applying molecular biology techniques that the genetic characteristics and portions of the genomic sequences of the virus could be identified; these techniques included the randomly primed PCR technique which obtained the limited sequence data needed to reveal a clear relationship between this new virus and the avian pneumovirus.[5] It was this close relationship to AMPV that gave rise to this new virus being named human metapneumovirus[5] to reflect both its identity as a metapneumovirus and its use of humans as a host organism.
Epidemiology
editHMPV was responsible for 12% of cases of acute respiratory tract illness in otherwise-healthy children in a US outpatient clinic[3] and 15% and 8% of cases (respectively) of community-acquired pneumonia requiring hospitalization in children under and over the age of 5 in the United States.[9] The virus is distributed worldwide and, in temperate regions, has a seasonal distribution generally following that of RSV and influenza virus during late winter and spring.[3][10] Serologic studies have shown that by the age of five, virtually all children worldwide have been exposed to the virus.[2][11][12][13] Despite near universal infection during early life, reinfections are common in older children and adults.[3][14][12][15] Human metapneumovirus may cause mild upper respiratory tract infection (the common cold). However, premature infants,[16] immunocompromised persons,[17][18][19][20] and older adults >65 years [15][21][22] are at risk for severe disease and hospitalization. In some studies of hospitalizations and emergency room visits, HMPV is nearly as common and as severe as influenza in older adults.[15][21][22][23] HMPV is associated with more severe disease in people with asthma[24][25][26][27] and adults with chronic obstructive pulmonary disease (COPD).[28][29][30] Numerous outbreaks of HMPV have been reported in long-term care facilities for children and adults, causing fatalities.[31][32][33][34][35]
Genome
editThe genomic organisation of HMPV is similar to RSV; however, HMPV lacks the non-structural genes, NS1 and NS2, and the HMPV antisense RNA genome contains eight open reading frames in slightly different gene order than RSV (viz. 3’-N-P-M-F-M2-SH-G-L-5’).[36] HMPV is genetically similar to the avian metapneumoviruses A, B and in particular type C. Phylogenetic analysis of HMPV has demonstrated the existence of two main genetic lineages termed subtype A and B containing within them the subgroups A1/A2 and B1/B2 respectively. Genotyping based on sequences of the F and G genes showed that subtype B was associated with increased cough duration and increased general respiratory systems compared to HMPV-A.[37]
Life cycle and reproduction
edithMPV is estimated to have a 3–6 day incubation period and is often most active during the later winter and spring seasons in temperate climates, overlapping with the RSV and influenza seasons and possibly allowing for repeated infection.[6] But because it is still a relatively new virus and has not yet been researched very heavily, hMPV and its replication cycle still have a lot of mystery surrounding them. However, researchers have been able to elucidate some principal steps of hMPV's replication cycle, basing their approach and experimentation on the current knowledge we have of the viral life cycles and reproductive measures of the rest of the Paramyxoviridae family.[38]
With that being said, it has been determined that the first step of the hMPV replication cycle is attachment to the host cell, specifically the epithelial cells of the respiratory tract, using the G protein.[8][38] This G protein contains a hydrophobic region that acts as an uncleaved signal peptide and a membrane anchor to facilitate its binding; however, because recombinant viruses that lack the G protein have still been able to replicate in vitro and in vivo, it seems that attachment via the G protein is not required for rest of the replication cycle.[8]
Next in the cycle is the fusion of the viral and host membranes which is likely mediated by the F protein.[8][38] Though the fusion mechanism is very similar to that of other Paramyxoviridae family members and involves conformational changes of the F protein, the mechanism for hMPV does not depend on the G protein for fusion like its family members, showing consistency with the previously mentioned idea that the G protein is not necessary for subsequent steps of the hMPV replication cycle.[8][38] Moreover, the fusion function of the F protein has been proven by its ability to bind to host cells via integrin αvβ1 using an Arginine-Glycine-Aspartate (RGD) motif, which is speculated to be the trigger for membrane fusion events.[8] One main difference between hMPV and other Paramyxoviridae viruses’ fusion mechanisms though is that hMPV's fusion events occur at acidic pH levels while other viruses’ fusion events occur at neutral pH levels; however, more research needs to be conducted in this area to get a better understanding of what is different about the hMPV fusion mechanism and why.[38] Although its specific function is uncertain, it is important to note the presence of the SH glycoprotein which seemingly does not have any effects on replication kinetics, cytopathic effects, or plaque formation of hMPV.[38]
After fusion, the viral ribonucleoprotein (RNP) containing negative-sense viral RNA (vRNA) genome is released into the cytoplasm and acts as a template for mRNA and antigenomic cRNA synthesis.[8] From here, most of our knowledge about hMPV transcription is derived from what we already know about RSV and other Paramyxoviridae viruses, including that leader and trailer sequences in the genome are partially complementary and act as promoters for transcription.[8] We see that proteins N, P, and L dissociate from the vRNA and bind to each other to form the polymerase complex so that the genomic RNA can act as a matrix for viral transcription and replication in the cytoplasm.[38] The final step in the replication process of hMPV that is relatively certain is the journeying of the envelope glycoproteins (F, G, and SH) to zones of membranous accumulation via the Golgi apparatus to be exposed at the surface of infected cells.[38] This allows infected cells to merge with adjacent cells through the action of viral fusion proteins on the surface, effectively spreading the virus's genome.[38] The rest of the replication cycle following RNA and viral protein synthesis are unclear and require further research.[8]
Virology
editHMPV infects airway epithelial cells in the nose and lung. HMPV is thought to attach to the target cell via the glycoprotein (G) protein interactions with heparan sulfate and other glycosaminoglycans. The HMPV fusion (F) protein encodes an RGD (Arg-Gly-Asp) motif that engages RGD-binding integrins as cellular receptors,[39][40][41][42] then mediates fusion of the cell membrane and viral envelope in a pH-independent fashion, likely within endosomes.[43][44] HMPV then induces the response of chemokines and cytokines such as IL-6, IFN-alpha, TNF-alpha, IL-2, and macrophage inflammatory proteins, which in turn leads to peribronchiolar and perivascular infiltration and inflammation.[45]
Detection
editThe identification of HMPV has predominantly relied on reverse-transcriptase polymerase chain reaction (RT-PCR) technology to amplify directly from RNA extracted from respiratory specimens. Alternative more cost-effective approaches to the detection of HMPV by nucleic acid-based approaches have been employed and these include:
- detection of hMPV antigens in nasopharyngeal secretions by immunofluorescent-antibody test
- the use of immunofluorescence staining with monoclonal antibodies to detect HMPV in nasopharyngeal secretions and shell vial cultures
- immunofluorescence assays for detection of hMPV-specific antibodies
- the use of polyclonal antibodies and direct isolation in cultured cells.
Distribution and hosts
editThough hMPV was first discovered and identified in 2001, serological studies showed that hMPV, or a close relative of it, had already been circulating for at least 50 years.[5][46] From this information, it is clear that the virus had not just “jumped” from birds, or some other animal reservoir, to humans shortly before its discovery.[5]
So far, peak infection from hMPV in the northern hemisphere is in late winter and early spring, but it can be found globally across all continents[46] and its distribution is very complex and dynamic.[5] Researchers have found that hMPV is mostly localized and can differ significantly from community to community, allowing for the possibility of the strain in one location one year to be most similar to the strain in a different location the next year.[5] This phenomenon has actually been recorded with the virus strains in Australia in 2001; in France in 2000 and 2002; in Canada in 1999, 2000, 2001, and 2002; in Israel in 2002; and in the Netherlands in 2001 all being very closely related based on their F gene sequences.[5] There are at least two major genotypes of hMPV (A and B) that circulate during community outbreaks and each genotype has two of its own,[5] but as of now, it seems that no one strain is dominant over the others and none of them are known to cause varying levels of severity.[46]
hMPV is most likely spread from infected individuals to others through 1. secretions from coughing and sneezing, 2. close personal contact (ex. touching, shaking hands, etc), and 3. touching objects with viruses on them then touching your mouth, nose, or eyes.[6] Development of a reliable antiviral therapy treatment or vaccine to prevent the spread of hMPV has yet to occur, but there does seem to be promising developments in that area.[5][6] In some vaccine trials, researchers have observed how a live recombinant human parainfluenza virus that contains the hMPV F gene can induce hMPV-specific antibodies and can protect experimental animals from hMPV.[5] Another similar study demonstrated how a chimeric bovine/human parainfluenza virus 3 expressing the hMPV F gene allows for neutralizing antibodies against both parainfluenza and hMPV.[5] However promising these results and trials may seem, it is important to note that these experiments have limitations including their small-population animal models.[5] Overall, while vaccines and antiviral therapy treatments are in the works, the biggest difficulty that researchers face at the moment is the limited data available about the development of hMPV in the natural host.[5]
Transmission
editThere are no conclusive studies to date; however, it is likely that transmission occurs by contact with contaminated secretions, via droplet, aerosol, or fomite vectors. Hospital-acquired infections with human metapneumovirus have been reported.[47] HMPV has been shown to circulate during fall and winter months with alternating predominance of a single subtype each year.[37]
Treatment
editNo treatment is yet known,[48] but ribavirin has shown effectiveness in an animal model.[49]
American pharmaceutical corporation Moderna has conducted a clinical trial for a candidate modRNA vaccine against metapneumovirus.[50] As of October 2019, the vaccine candidate has passed through phase I, with reports that the vaccine is well-tolerated at all dose levels at two months, and provokes an immune response which boosts the production of neutralising antibodies.[51]
Evolution
editHuman metapneumovirus was first reported in 2001 and avian metapneumovirus in the 1970s. There are at least four lineages of human metapneumovirus—A1, A2, B1 and B2. Avian metapneumovirus has been divided into four subgroups—A, B, C and D. Bayesian estimates suggest that human metapneumovirus emerged 119–133 years ago and diverged from avian metapneumovirus around 1800.[52]
References
edit- ^ a b "ICTV Online (10th) Report".
- ^ a b van den Hoogen, Bernadette G.; Jong, Jan C. de; Groen, Jan; Kuiken, Thijs; Groot, Ronald de; Fouchier, Ron A.M.; Osterhaus, Albert D.M.E. (2001). "A newly discovered human pneumovirus isolated from young children with respiratory tract disease". Nature Medicine. 7 (6): 719–724. doi:10.1038/89098. PMC 7095854. PMID 11385510.
- ^ a b c d Williams, John V.; Harris, Paul A.; Tollefson, Sharon J.; Halburnt-Rush, Lisa L.; Pingsterhaus, Joyce M.; Edwards, Kathryn M.; Wright, Peter F.; Crowe, James E. Jr. (2004-01-29). "Human Metapneumovirus and Lower Respiratory Tract Disease in Otherwise Healthy Infants and Children". New England Journal of Medicine. 350 (5): 443–450. doi:10.1056/nejmoa025472. ISSN 0028-4793. PMC 1831873. PMID 14749452.
- ^ Amarasinghe, Gaya K.; Bào, Yīmíng; Basler, Christopher F.; Bavari, Sina; Beer, Martin; Bejerman, Nicolás; Blasdell, Kim R.; Bochnowski, Alisa; Briese, Thomas (2017-04-07). "Taxonomy of the order Mononegavirales: update 2017". Archives of Virology. 162 (8): 2493–2504. doi:10.1007/s00705-017-3311-7. PMC 5831667. PMID 28389807.
- ^ a b c d e f g h i j k l m n o Kahn, Jeffrey S. (July 2006). "Epidemiology of Human Metapneumovirus". Clinical Microbiology Reviews. 19 (3): 546–557. doi:10.1128/cmr.00014-06. PMC 1539100. PMID 16847085.
- ^ a b c d "Human Metapneumovirus". Centers for Disease Control and Prevention. 13 April 2023.
- ^ "Human Metapneumovirus (HMPV): Causes & Treatment". Cleveland Clinic.
- ^ a b c d e f g h i Schildgen, Verena; Van Den Hoogen, Bernadette; Fouchier, Ron; Tripp, Ralph A.; Alvarez, Rene; Manoha, Catherine; Williams, John; Schildgen, Oliver (Oct 2011). "Human Metapneumovirus: Lessons Learned over the First Decade". Clinical Microbiology Reviews. 24 (4): 734–754. doi:10.1128/cmr.00015-11. PMC 3194831. PMID 21976607.
- ^ Jain, Seema; Williams, Derek J.; Arnold, Sandra R.; Ampofo, Krow; Bramley, Anna M.; Reed, Carrie; Stockmann, Chris; Anderson, Evan J.; Grijalva, Carlos G. (2015-02-25). "Community-Acquired Pneumonia Requiring Hospitalization among U.S. Children". New England Journal of Medicine. 372 (9): 835–845. doi:10.1056/nejmoa1405870. PMC 4697461. PMID 25714161.
- ^ Williams, John V.; Wang, Chiaoyin K.; Yang, Chin-Fen; Tollefson, Sharon J.; House, Frances S.; Heck, Josh M.; Chu, Marla; Brown, Jennifer B.; Lintao, Linda D. (2006-02-01). "The Role of Human Metapneumovirus in Upper Respiratory Tract Infections in Children: A 20-Year Experience". The Journal of Infectious Diseases. 193 (3): 387–395. doi:10.1086/499274. ISSN 0022-1899. PMC 1586246. PMID 16388486.
- ^ Leung, Jessica; Esper, Frank; Weibel, Carla; Kahn, Jeffrey S. (2005-03-01). "Seroepidemiology of Human Metapneumovirus (hMPV) on the Basis of a Novel Enzyme-Linked Immunosorbent Assay Utilizing hMPV Fusion Protein Expressed in Recombinant Vesicular Stomatitis Virus". Journal of Clinical Microbiology. 43 (3): 1213–1219. doi:10.1128/jcm.43.3.1213-1219.2005. ISSN 0095-1137. PMC 1081231. PMID 15750086.
- ^ a b Pavlin, Julie A.; Hickey, Andrew C.; Ulbrandt, Nancy; Chan, Yee-Peng; Endy, Timothy P.; Boukhvalova, Marina S.; Chunsuttiwat, Supamit; Nisalak, Ananda; Libraty, Daniel H. (2008-09-15). "Human Metapneumovirus Reinfection among Children in Thailand Determined by ELISA Using Purified Soluble Fusion Protein". The Journal of Infectious Diseases. 198 (6): 836–842. doi:10.1086/591186. ISSN 0022-1899. PMC 2648801. PMID 18680407.
- ^ Dunn, Sarah R.; Ryder, Alex B.; Tollefson, Sharon J.; Xu, Meng; Saville, Benjamin R.; Williams, John V. (2013-10-01). "Seroepidemiologies of Human Metapneumovirus and Respiratory Syncytial Virus in Young Children, Determined with a New Recombinant Fusion Protein Enzyme-Linked Immunosorbent Assay". Clinical and Vaccine Immunology. 20 (10): 1654–1656. doi:10.1128/cvi.00750-12. ISSN 1556-6811. PMC 3807191. PMID 23945161.
- ^ Howard, Leigh M.; Edwards, Kathryn M.; Zhu, Yuwei; Griffin, Marie R.; Weinberg, Geoffrey A.; Szilagyi, Peter G.; Staat, Mary A.; Payne, Daniel C.; Williams, John V. (2017). "Clinical Features of Human Metapneumovirus Infection in Ambulatory Children Aged 5–13 Years". Journal of the Pediatric Infectious Diseases Society. 7 (2): 165–168. doi:10.1093/jpids/pix012. PMC 5954304. PMID 28369564.
- ^ a b c Falsey, Ann R.; Erdman, Dean; Anderson, Larry J.; Walsh, Edward E. (2003-03-01). "Human Metapneumovirus Infections in Young and Elderly Adults". The Journal of Infectious Diseases. 187 (5): 785–790. doi:10.1086/367901. ISSN 0022-1899. PMID 12599052.
- ^ Williams, John V.; Maitre, Nathalie (2016-07-28). "Human metapneumovirus in the preterm neonate: current perspectives". Research and Reports in Neonatology. 6: 41–49. doi:10.2147/rrn.s76270. PMC 5120728. PMID 27891060.
- ^ Shahda, S.; Carlos, W.g.; Kiel, P.j.; Khan, B.a.; Hage, C.a. (2011-06-01). "The human metapneumovirus: a case series and review of the literature". Transplant Infectious Disease. 13 (3): 324–328. doi:10.1111/j.1399-3062.2010.00575.x. ISSN 1399-3062. PMC 3107511. PMID 21631655.
- ^ Chu, Helen Y.; Renaud, Christian; Ficken, Elle; Thomson, Blythe; Kuypers, Jane; Englund, Janet A. (2014-12-01). "Respiratory Tract Infections Due to Human Metapneumovirus in Immunocompromised Children". Journal of the Pediatric Infectious Diseases Society. 3 (4): 286–293. doi:10.1093/jpids/piu100. ISSN 2048-7193. PMC 4240341. PMID 25419459.
- ^ Seo, Sachiko; Gooley, Ted A.; Kuypers, Jane M.; Stednick, Zachary; Jerome, Keith R.; Englund, Janet A.; Boeckh, Michael (2016-07-15). "Human Metapneumovirus Infections Following Hematopoietic Cell Transplantation: Factors Associated With Disease Progression". Clinical Infectious Diseases. 63 (2): 178–185. doi:10.1093/cid/ciw284. ISSN 1058-4838. PMC 4928387. PMID 27143659.
- ^ Shah, Dimpy P.; Shah, Pankil K.; Azzi, Jacques M.; Chaer, Firas El; Chemaly, Roy F. (2016). "Human metapneumovirus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: A systematic review". Cancer Letters. 379 (1): 100–106. doi:10.1016/j.canlet.2016.05.035. PMC 4935561. PMID 27260872.
- ^ a b Walsh, Edward E.; Peterson, Derick R.; Falsey, Ann R. (2008-12-08). "Human Metapneumovirus Infections in Adults: Another Piece of the Puzzle". Archives of Internal Medicine. 168 (22): 2489–2496. doi:10.1001/archinte.168.22.2489. ISSN 0003-9926. PMC 2783624. PMID 19064834.
- ^ a b Widmer, Kyle; Zhu, Yuwei; Williams, John V.; Griffin, Marie R.; Edwards, Kathryn M.; Talbot, H. Keipp (2012-07-01). "Rates of Hospitalizations for Respiratory Syncytial Virus, Human Metapneumovirus, and Influenza Virus in Older Adults". The Journal of Infectious Diseases. 206 (1): 56–62. doi:10.1093/infdis/jis309. ISSN 0022-1899. PMC 3415933. PMID 22529314.
- ^ Widmer, Kyle; Griffin, Marie R.; Zhu, Yuwei; Williams, John V.; Talbot, H. Keipp (2014-05-01). "Respiratory syncytial virus- and human metapneumovirus-associated emergency department and hospital burden in adults". Influenza and Other Respiratory Viruses. 8 (3): 347–352. doi:10.1111/irv.12234. ISSN 1750-2659. PMC 3984605. PMID 24512531.
- ^ Williams, John V.; Crowe, James E.; Enriquez, Rachel; Minton, Patricia; Peebles, R. Stokes; Hamilton, Robert G.; Higgins, Stanley; Griffin, Marie; Hartert, Tina V. (2005-10-01). "Human Metapneumovirus Infection Plays an Etiologic Role in Acute Asthma Exacerbations Requiring Hospitalization in Adults". The Journal of Infectious Diseases. 192 (7): 1149–1153. doi:10.1086/444392. ISSN 0022-1899. PMC 1476781. PMID 16136455.
- ^ Williams, John V.; Tollefson, Sharon J.; Heymann, Peter W.; Carper, Holliday T.; Patrie, James; Crowe Jr., James E. (2005). "Human metapneumovirus infection in children hospitalized for wheezing". Journal of Allergy and Clinical Immunology. 115 (6): 1311–1312. doi:10.1016/j.jaci.2005.02.001. PMC 1476700. PMID 15940152.
- ^ García-García, M.l.; Calvo, C.; Casas, I.; Bracamonte, T.; Rellán, A.; Gozalo, F.; Tenorio, T.; Pérez-Breña, P. (2007-05-01). "Human metapneumovirus bronchiolitis in infancy is an important risk factor for asthma at age 5". Pediatric Pulmonology. 42 (5): 458–464. doi:10.1002/ppul.20597. ISSN 1099-0496. PMID 17427899. S2CID 2395811.
- ^ Khetsuriani, Nino; Kazerouni, N. Neely; Erdman, Dean D.; Lu, Xiaoyan; Redd, Stephen C.; Anderson, Larry J.; Teague, W. Gerald (2007). "Prevalence of viral respiratory tract infections in children with asthma". Journal of Allergy and Clinical Immunology. 119 (2): 314–321. doi:10.1016/j.jaci.2006.08.041. PMC 7112359. PMID 17140648.
- ^ Vicente, Diego; Montes, Milagrosa; Cilla, Gustavo; Pérez-Trallero, Emilio (July 2004). "Human Metapneumovirus and Chronic Obstructive Pulmonary Disease". Emerging Infectious Diseases. 10 (7): 1338–1339. doi:10.3201/eid1007.030633. ISSN 1080-6040. PMC 3323314. PMID 15338546.
- ^ Martinello, Richard A.; Esper, Frank; Weibel, Carla; Ferguson, David; Landry, Marie L.; Kahn, Jeffrey S. (2006). "Human metapneumovirus and exacerbations of chronic obstructive pulmonary disease". Journal of Infection. 53 (4): 248–254. doi:10.1016/j.jinf.2005.11.010. PMC 7112509. PMID 16412516.
- ^ Kan-o, Keiko; Ramirez, Ruben; MacDonald, Martin I.; Rolph, Michael; Rudd, Penny A.; Spann, Kirsten M.; Mahalingam, Suresh; Bardin, Philip G.; Thomas, Belinda J. (2017-05-15). "Human Metapneumovirus Infection in Chronic Obstructive Pulmonary Disease: Impact of Glucocorticosteroids and Interferon". The Journal of Infectious Diseases. 215 (10): 1536–1545. doi:10.1093/infdis/jix167. ISSN 0022-1899. PMID 28379462.
- ^ Boivin, Guy; Serres, Gaston De; Hamelin, Marie-Eve; Côté, Stéphanie; Argouin, Marco; Tremblay, Geneviève; Maranda-Aubut, Renée; Sauvageau, Chantal; Ouakki, Manale (2007-05-01). "An Outbreak of Severe Respiratory Tract Infection Due to Human Metapneumovirus in a Long-Term Care Facility". Clinical Infectious Diseases. 44 (9): 1152–1158. doi:10.1086/513204. ISSN 1058-4838. PMID 17407031.
- ^ Louie, Janice K.; Schnurr, David P.; Pan, Chao-Yang; Kiang, David; Carter, Connie; Tougaw, Sandra; Ventura, Jean; Norman, Agnes; Belmusto, Vivian (2007-09-01). "A Summer Outbreak of Human Metapneumovirus Infection in a Long-Term-Care Facility". The Journal of Infectious Diseases. 196 (5): 705–708. doi:10.1086/519846. ISSN 0022-1899. PMID 17674312.
- ^ Neu, Natalie; Plaskett, Theresa; Hutcheon, Gordon; Murray, Meghan; Southwick, Karen L.; Saiman, Lisa (June 2012). "Epidemiology of Human Metapneumovirus in a Pediatric Long-Term Care Facility". Infection Control & Hospital Epidemiology. 33 (6): 545–550. doi:10.1086/665727. ISSN 0899-823X. PMID 22561708. S2CID 2132679.
- ^ "Outbreaks of Human Metapneumovirus in Two Skilled Nursing Facilities — West Virginia and Idaho, 2011–2012". www.cdc.gov. Retrieved 2017-09-16.
- ^ Yang, Zifeng; Suzuki, Akira; Watanabe, Oshi; Okamoto, Michiko; Ohmi, Akira; Huang, Wenbo; Nishimura, Hidekazu (2014). "Outbreak of human metapneumovirus infection in a severe motor-and-intellectual disabilities ward in Japan". Japanese Journal of Infectious Diseases. 67 (4): 318–321. doi:10.7883/yoken.67.318. ISSN 1884-2836. PMID 25056083.
- ^ van den Hoogen, Bernadette G.; Bestebroer, Theo M.; Osterhaus, Albert D. M. E.; Fouchier, Ron A. M. (2002-03-30). "Analysis of the genomic sequence of a human metapneumovirus". Virology. 295 (1): 119–132. doi:10.1006/viro.2001.1355. hdl:1765/3864. ISSN 0042-6822. PMID 12033771.
- ^ a b Perchetti, GA; Wilcox, N; Chu, HY; Katz, J; Khatry, SK; LeClerq, SC; Tielsch, JM; Jerome, KR; Englund, JA; Kuypers, J (November 2020). "Human Metapneumovirus Infection and Genotyping of Infants in Rural Nepal". Journal of the Pediatric Infectious Diseases Society. 10 (4): 408–416. doi:10.1093/jpids/piaa118. PMID 33137178.
- ^ a b c d e f g h i Feuillet, F.; Lina, B.; Rosa-Calatrava, M.; Boivin, G. (Feb 2012). "Ten years of human metapneumovirus research". Journal of Clinical Virology. 53 (2): 97–105. doi:10.1016/j.jcv.2011.10.002. PMID 22074934.
- ^ Cseke, G.; Maginnis, M. S.; Cox, R. G.; Tollefson, S. J.; Podsiad, A. B.; Wright, D. W.; Dermody, T. S.; Williams, J. V. (2009). "Integrin v 1 promotes infection by human metapneumovirus". Proceedings of the National Academy of Sciences. 106 (5): 1566–1571. doi:10.1073/pnas.0801433106. PMC 2629439. PMID 19164533.
- ^ Chang, A.; Masante, C.; Buchholz, U. J.; Dutch, R. E. (2012). "Human Metapneumovirus (HMPV) Binding and Infection Are Mediated by Interactions between the HMPV Fusion Protein and Heparan Sulfate". Journal of Virology. 86 (6): 3230–3243. doi:10.1128/JVI.06706-11. PMC 3302303. PMID 22238303.
- ^ Cox, R. G.; Livesay, S. B.; Johnson, M.; Ohi, M. D.; Williams, J. V. (2012). "The Human Metapneumovirus Fusion Protein Mediates Entry via an Interaction with RGD-Binding Integrins". Journal of Virology. 86 (22): 12148–12160. doi:10.1128/JVI.01133-12. PMC 3486500. PMID 22933271.
- ^ Wei, Y.; Zhang, Y.; Cai, H.; Mirza, A. M.; Iorio, R. M.; Peeples, M. E.; Niewiesk, S.; Li, J. (2014). "Roles of the Putative Integrin-Binding Motif of the Human Metapneumovirus Fusion (F) Protein in Cell-Cell Fusion, Viral Infectivity, and Pathogenesis". Journal of Virology. 88 (8): 4338–4352. doi:10.1128/JVI.03491-13. PMC 3993731. PMID 24478423.
- ^ Schowalter, R. M.; Smith, S. E.; Dutch, R. E. (2006). "Characterization of Human Metapneumovirus F Protein-Promoted Membrane Fusion: Critical Roles for Proteolytic Processing and Low pH". Journal of Virology. 80 (22): 10931–10941. doi:10.1128/JVI.01287-06. PMC 1642150. PMID 16971452.
- ^ Cox, Reagan G.; Mainou, Bernardo A.; Johnson, Monika; Hastings, Andrew K.; Schuster, Jennifer E.; Dermody, Terence S.; Williams, John V. (2015). "Human Metapneumovirus is Capable of Entering Cells by Fusion with Endosomal Membranes". PLOS Pathogens. 11 (12): e1005303. doi:10.1371/journal.ppat.1005303. PMC 4667933. PMID 26629703.
- ^ Uddin, Sanaa; Thomas, Meagan (2023), "Human Metapneumovirus", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 32809745, retrieved 2023-11-18
- ^ a b c Uddin, Sanaa; Thomas, Meagan (July 18, 2022). "Human Metapneumovirus". StatPearls [Internet]. PMID 32809745.
- ^ Peiris, JS; Tang, WH; Chan, KH; Khong, PL; Guan, Y; Lau, YL; Chiu, SS (June 2003). "Children with respiratory disease associated with metapneumovirus in Hong Kong". Emerging Infectious Diseases. 9 (6): 628–633. doi:10.3201/eid0906.030009. PMC 3000155. PMID 12781000.
- ^ Bao X, Liu T, Shan Y, Li K, Garofalo RP, Casola A (May 2008). Baric RS (ed.). "Human Metapneumovirus Glycoprotein G Inhibits Innate Immune Responses". PLOS Pathog. 4 (5): e1000077. doi:10.1371/journal.ppat.1000077. PMC 2386556. PMID 18516301.
- ^ Deffrasnes C, Hamelin ME, Boivin G (April 2007). "Human metapneumovirus". Semin Respir Crit Care Med. 28 (2): 213–21. doi:10.1055/s-2007-976493. PMID 17458775. S2CID 21308968.
- ^ https://trials.modernatx.com/trials/NCT03392389 [dead link]
- ^ "Moderna to Present Data from Two of Its Prophylactic mRNA Vaccines at IDWeek 2019" (Press release). 2 October 2019.
- ^ de Graaf M, Osterhaus AD, Fouchier RA, Holmes EC (2008). "Evolutionary dynamics of human and avian metapneumoviruses". J. Gen. Virol. 89 (Pt 12): 2933–42. doi:10.1099/vir.0.2008/006957-0. PMID 19008378.
External links
edit- ICTV Virus Taxonomy Profile: Pneumoviridae (December 2017)
- hMPV EIA kit (Biotrin, archived 13 February 2007)
- Human Metapneumovirus, hMPV (Biotrin, archived 11 September 2007)
- Virus Pathogen Database and Analysis Resource (ViPR): Paramyxoviridae[dead link]