Metabolic acidosis is a serious electrolyte disorder characterized by an imbalance in the body's acid-base balance. Metabolic acidosis has three main root causes: increased acid production, loss of bicarbonate, and a reduced ability of the kidneys to excrete excess acids.[5] Metabolic acidosis can lead to acidemia, which is defined as arterial blood pH that is lower than 7.35.[6] Acidemia and acidosis are not mutually exclusive – pH and hydrogen ion concentrations also depend on the coexistence of other acid-base disorders; therefore, pH levels in people with metabolic acidosis can range from low to high.
| Metabolic acidosis | |
|---|---|
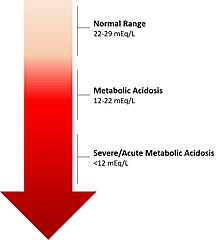 | |
| The calculated level of bicarbonate in the blood (HCO3−) reflects the severity of acidosis. | |
| Specialty | Nephrology |
| Complications | Acute: poor morbidity and mortality outcomes; Chronic: adverse outcomes on kidney function, musculoskeletal system, possible cardiovascular effects |
| Types | Acute Metabolic Acidosis Chronic Metabolic Acidosis |
| Causes | Acute: Excessive amounts of organic acids; Chronic: Impaired kidney function |
| Diagnostic method | Level of bicarbonate (HCO3-) in the blood |
| Treatment | Acute: Mitigation of the underlying cause for the metabolic problem, such as administration of insulin in cases of diabetic ketoacidosis or restoration of effective circulating intravascular volume in cases of lactic acidosis. The administration of IV bicarbonate, although intellectually appealing, is rarely indicated or administered Chronic: Diet rich in fruits and vegetables, oral alkali therapy[1] |
| Frequency | Acute: Most often presented during critical illnesses, and hospitalizations: incidence ranging 14–42%.[2][3] Chronic: Highly prevalent in people with Chronic Kidney Disease: 9.4% CKD Stage 3a; 18.1% CKD Stage 3b; 31.5% CKD Stage 4 and 5 [4] |
Acute metabolic acidosis, lasting from minutes to several days, often occurs during serious illnesses or hospitalizations, and is generally caused when the body produces an excess amount of organic acids (ketoacids in ketoacidosis, or lactic acid in lactic acidosis). A state of chronic metabolic acidosis, lasting several weeks to years, can be the result of impaired kidney function (chronic kidney disease) and/or bicarbonate wasting. The adverse effects of acute versus chronic metabolic acidosis also differ, with acute metabolic acidosis impacting the cardiovascular system in hospital settings, and chronic metabolic acidosis affecting muscles, bones, kidney and cardiovascular health.[7]
Signs and symptoms
editAcute metabolic acidosis
editSymptoms are not specific, and diagnosis can be difficult unless patients present with clear indications for blood gas sampling. Symptoms may include palpitations, headache, altered mental status such as severe anxiety due to hypoxia, decreased visual acuity, nausea, vomiting, abdominal pain, altered appetite and weight gain, muscle weakness, bone pain, and joint pain. People with acute metabolic acidosis may exhibit deep, rapid breathing called Kussmaul respirations which is classically associated with diabetic ketoacidosis.[8] Rapid deep breaths increase the amount of carbon dioxide exhaled, thus lowering the serum carbon dioxide levels, resulting in some degree of compensation. Overcompensation via respiratory alkalosis to form an alkalemia does not occur.[citation needed]
Extreme acidemia can also lead to neurological and cardiac complications:[citation needed]
- Neurological: lethargy, stupor, coma, seizures
- Cardiac: Abnormal heart rhythms (e.g., ventricular tachycardia) and decreased response to epinephrine, both leading to low blood pressure
Physical examination can occasionally reveal signs of the disease, but is often otherwise normal. Cranial nerve abnormalities are reported in ethylene glycol poisoning, and retinal edema can be a sign of methanol intoxication.[citation needed]
Chronic metabolic acidosis
editChronic metabolic acidosis has non-specific clinical symptoms but can be readily diagnosed by testing serum bicarbonate levels in patients with chronic kidney disease (CKD) as part of a comprehensive metabolic panel. Patients with CKD Stages G3–G5 should be routinely screened for metabolic acidosis.[9][10]
Diagnostic approach and causes
editMetabolic acidosis results in a reduced serum pH that is due to metabolic and not respiratory dysfunction. Typically the serum bicarbonate concentration will be <22 mEq/L, below the normal range of 22 to 29 mEq/L, the standard base will be more negative than -2 (base deficit) and the pCO2 will be reduced as a result of hyperventilation in an attempt to restore the pH closer to normal. Occasionally in a mixed acid-base disorder where metabolic acidosis is not the primary disorder present, the pH may be normal or high.[5] In the absence of chronic respiratory alkalosis, metabolic acidosis can be clinically diagnosed by analysis of the calculated serum bicarbonate level.[citation needed]
Causes
editGenerally, metabolic acidosis occurs when the body produces too much acid (e.g., lactic acidosis, see below section), there is a loss of bicarbonate from the blood, or when the kidneys are not removing enough acid from the body.[citation needed]
Chronic metabolic acidosis is most often caused by a decreased capacity of the kidneys to excrete excess acids through renal ammoniagenesis. The typical Western diet generates 75–100 mEq of acid daily,[11] and individuals with normal kidney function increase the production of ammonia to get rid of this dietary acid. As kidney function declines, the tubules lose the ability to excrete excess acid, and this results in buffering of acid using serum bicarbonate, as well as bone and muscle stores.[12]
There are many causes of acute metabolic acidosis, and thus it is helpful to group them by the presence or absence of a normal anion gap.[13]
Increased anion gap
Causes of increased anion gap include:
- Lactic acidosis[14]
- Ketoacidosis (e.g., Diabetic, alcoholic, or starvation)[15]
- Chronic kidney failure[16]
- 5-oxoprolinemia due to long-term ingestion of high-doses of acetaminophen with glutathione depletion[17] (often seen with sepsis, liver failure, kidney failure, or malnutrition[citation needed])
- Intoxication:
- Salicylates, methanol, ethylene glycol[15]
- Organic acids, paraldehyde, ethanol, formaldehyde[18]
- Carbon monoxide, cyanide, ibuprofen, metformin[19]
- Propylene glycol (metabolized to L and D-lactate and is often found in infusions for certain intravenous medications used in the intensive care unit)[20]
- Massive rhabdomyolysis[21]
- Isoniazid, iron, phenelzine, tranylcypromine, valproic acid, verapamil[22]
- Topiramate
- Sulfates[23]
Normal anion gap
Causes of normal anion gap include:[24]
- Inorganic acid addition
- Infusion/ingestion of HCl, NH
4Cl
- Infusion/ingestion of HCl, NH
- Gastrointestinal base loss
- Diarrhea
- Small bowel fistula/drainage
- Surgical diversion of urine into gut loops
- Renal base loss/acid retention:
- Hyperalimentation
- Addison disease
- Acetazolamide
- Spironolactone
- Saline infusion
To distinguish between the main types of metabolic acidosis, a clinical tool called the anion gap is very useful. The anion gap is calculated by subtracting the sum of the serum concentrations of major anions, chloride and bicarbonate, from the serum concentration of the major cation, sodium. (The serum potassium concentration may be added to the calculation, but this merely changes the normal reference range for what is considered a normal anion gap)
Because the concentration of serum sodium is greater than the combined concentrations of chloride and bicarbonate an 'anion gap' is noted. In reality serum is electoneutral because of the presence of other minor cations (potassium, calcium and magnesium) and anions (albumin, sulphate and phosphate) that are not measured in the equation that calculates the anion gap.[citation needed]
The normal value for the anion gap is 8–16 mmol/L (12±4). An elevated anion gap (i.e. > 16 mmol/L) indicates the presence of excess 'unmeasured' anions, such as lactic acid in anaerobic metabolism resulting from tissue hypoxia, glycolic and formic acid produced by the metabolism of toxic alcohols, ketoacids produced when acetyl-CoA undergoes ketogenesis rather than entering the tricarboxylic (Krebs) cycle, and failure of renal excretion of products of metabolism such as sulphates and phosphates.[citation needed]
Adjunctive tests are useful in determining the aetiology of a raised anion gap metabolic acidosis including detection of an osmolar gap indicative of the presence of a toxic alcohol, measurement of serum ketones indicative of ketoacidosis and renal function tests and urinanalysis to detect renal dysfunction.[citation needed]
Elevated protein (albumin, globulins) may theoretically increase the anion gap but high levels are not usually encountered clinically. Hypoalbuminaemia, which is frequently encountered clinically, will mask an anion gap. As a rule of thumb, a decrease in serum albumin by 1 G/L will decrease the anion gap by 0.25 mmol/L[citation needed]
Pathophysiology
editCompensatory mechanisms
editMetabolic acidosis is characterized by a low concentration of bicarbonate (HCO−
3), which can happen with increased generation of acids (such as ketoacids or lactic acid), excess loss of HCO−
3 by the kidneys or gastrointestinal tract, or an inability to generate sufficient HCO−
3.[25] Thus demonstrating the importance of maintaining balance between acids and bases in the body for maintaining optimal functioning of organs, tissues and cells.[citation needed]
The body regulates the acidity of the blood by four buffering mechanisms.[citation needed]
- Bicarbonate buffering system
- Intracellular buffering by absorption of hydrogen atoms by various molecules, including proteins, phosphates and carbonate in bone.
- Respiratory compensation. Hyperventilation will cause more carbon dioxide to be removed from the body and thereby increases pH.
- Kidney compensation
Buffer
editThe decreased bicarbonate that distinguishes metabolic acidosis is therefore due to two separate processes: the buffer (from water and carbon dioxide) and additional renal generation. The buffer reactions are:
The Henderson–Hasselbalch equation mathematically describes the relationship between blood pH and the components of the bicarbonate buffering system: where pKa ≈ 6.1. In clinical practice, the CO2 concentration is usually determined via Henry's law from PaCO2, the CO2 partial pressure in arterial blood:
For example, blood gas machines usually determine bicarbonate concentrations from measured pH and PaCO2 values. Mathematically, the algorithm substitutes the Henry's law formula into the Henderson-Hasselbach equation and then rearranges: At sea level, normal numbers might be pH ≈ 7.4 and PaCO2 ≈ 40 mmHg; these then imply
Consequences
editAcute metabolic acidosis
editAcute metabolic acidosis most often occurs during hospitalizations, and acute critical illnesses. It is often associated with poor prognosis, with a mortality rate as high as 57% if the pH remains untreated at 7.20.[2] At lower pH levels, acute metabolic acidosis can lead to impaired circulation and end organ function.[citation needed]
Chronic metabolic acidosis
editChronic metabolic acidosis commonly occurs in people with chronic kidney disease (CKD) with an eGFR of less than 45 ml/min/1.73m2, most often with mild to moderate severity; however, metabolic acidosis can manifest earlier on in the course of CKD. Multiple animal and human studies have shown that metabolic acidosis in CKD, given its chronic nature, has a profound adverse impact on cellular function, overall contributing to high morbidities in patients.
The most adverse consequences of chronic metabolic acidosis in people with CKD, and in particular, for those who have end-stage renal disease (ESRD), are detrimental changes to the bones and muscles.[26] Acid buffering leads to loss of bone density, resulting in an increased risk of bone fractures,[27] renal osteodystrophy,[28] and bone disease;[26] as well, increased protein catabolism leads to muscle wasting.[29][30] Furthermore, metabolic acidosis in CKD is also associated with a reduction in eGFR; it is both a complication of CKD, as well as an underlying cause of CKD progression.[31][32][33][34]
Treatment
editTreatment of metabolic acidosis depends on the underlying cause, and should target reversing the main process. When considering course of treatment, it is important to distinguish between acute versus chronic forms.[citation needed]
Acute metabolic acidosis
editBicarbonate therapy is generally administered In patients with severe acute acidemia (pH < 7.11), or with less severe acidemia (pH 7.1–7.2) who have severe acute kidney injury. Bicarbonate therapy is not recommended for people with less severe acidosis (pH ≥ 7.1), unless severe acute kidney injury is present. In the BICAR-ICU trial,[35] bicarbonate therapy for maintaining a pH >7.3 had no overall effect on the composite outcome of all-cause mortality and the presence of at least one organ failure at day 7. However, amongst the sub-group of patients with severe acute kidney injury, bicarbonate therapy significantly decreased the primary composite outcome, and 28-day mortality, along with the need for dialysis.[citation needed]
Chronic metabolic acidosis
editFor people with chronic kidney disease (CKD), treating metabolic acidosis slows the progression of CKD.[36] Dietary interventions for treatment of chronic metabolic acidosis include base-inducing fruits and vegetables that assist with reducing the urine net acid excretion, and increase TCO2. Recent research has also suggested that dietary protein restriction, through ketoanalogue-supplemented vegetarian very low protein diets are also a nutritionally safe option for correction of metabolic acidosis in people with CKD.[37]
Currently, the most commonly used treatment for chronic metabolic acidosis is oral bicarbonate. The NKF/KDOQI guidelines recommend starting treatment when serum bicarbonate levels are <22 mEq/L, in order to maintain levels ≥ 22 mEq/L.[9][10] Studies investigating the effects of oral alkali therapy demonstrated improvements in serum bicarbonate levels, resulting in a slower decline in kidney function, and reduction in proteinuria – leading to a reduction in the risk of progressing to kidney failure. However, side effects of oral alkali therapy include gastrointestinal intolerance, worsening edema, and worsening hypertension. Furthermore, large doses of oral alkali are required to treat chronic metabolic acidosis, and the pill burden can limit adherence.[38]
Veverimer (TRC 101) is a promising investigational drug designed to treat metabolic acidosis by binding with the acid in the gastrointestinal tract and removing it from the body through excretion in the feces, in turn decreasing the amount of acid in the body, and increasing the level of bicarbonate in the blood. Results from a Phase 3, double-blind placebo-controlled 12-week clinical trial in people with CKD and metabolic acidosis demonstrated that Veverimer effectively and safely corrected metabolic acidosis in the short-term,[39] and a blinded, placebo-controlled, 40-week extension of the trial assessing long-term safety, demonstrated sustained improvements in physical function and a combined endpoint of death, dialysis, or 50% decline in eGFR.[40]
See also
editReferences
edit- ^ Navaneethan, Sankar D.; Shao, Jun; Buysse, Jerry; Bushinsky, David A. (5 July 2019). "Effects of Treatment of Metabolic Acidosis in CKD: A Systematic Review and Meta-Analysis". Clinical Journal of the American Society of Nephrology. 14 (7): 1011–1020. doi:10.2215/CJN.13091118. PMC 6625635. PMID 31196951.
- ^ a b Kraut, Jeffrey A.; Madias, Nicolaos E. (4 September 2012). "Treatment of acute metabolic acidosis: a pathophysiologic approach". Nature Reviews Nephrology. 8 (10): 589–601. doi:10.1038/nrneph.2012.186. PMID 22945490. S2CID 34657707.
- ^ Jung, Boris; Rimmele, Thomas; Le Goff, Charlotte; Chanques, Gérald; Corne, Philippe; Jonquet, Olivier; Muller, Laurent; Lefrant, Jean-Yves; Guervilly, Christophe; Papazian, Laurent; Allaouchiche, Bernard; Jaber, Samir (2011). "Severe metabolic or mixed acidemia on intensive care unit admission: incidence, prognosis and administration of buffer therapy. A prospective, multiple-center study". Critical Care. 15 (5): R238. doi:10.1186/cc10487. PMC 3334789. PMID 21995879.
- ^ Inker, Lesley A.; Coresh, Josef; Levey, Andrew S.; Tonelli, Marcello; Muntner, Paul (1 December 2011). "Estimated GFR, Albuminuria, and Complications of Chronic Kidney Disease". Journal of the American Society of Nephrology. 22 (12): 2322–2331. doi:10.1681/ASN.2010111181. PMC 3279937. PMID 21965377.
- ^ a b Emmett, Michael; Szerlip, Harold. "Approach to the adult with metabolic acidosis".
- ^ Costanzo, Linda (2010). Physiology. Philadelphia, Pennsylvania: Elsevier. ISBN 978-1-4160-6216-5.
- ^ Kraut, Jeffrey A.; Madias, Nicolaos E. (2010-05-01). "Metabolic acidosis: pathophysiology, diagnosis and management". Nature Reviews Nephrology. 6 (5): 274–285. doi:10.1038/nrneph.2010.33. ISSN 1759-5061. PMID 20308999. S2CID 205512465.
- ^ Gallo de Moraes, Alice; Surani, Salim (2019-01-15). "Effects of diabetic ketoacidosis in the respiratory system". World Journal of Diabetes. 10 (1): 16–22. doi:10.4239/wjd.v10.i1.16. ISSN 1948-9358. PMC 6347653. PMID 30697367.
- ^ a b "National Kidney Foundation: K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease" (PDF). Am J Kidney Dis. 42 (Suppl 3): S1–S201.
- ^ a b "CKD Evaluation and Management – KDIGO". kdigo.org. Retrieved 2019-12-31.
- ^ Weaver, Connie M. (2013-05-06). "Potassium and Health123". Advances in Nutrition. 4 (3): 368S–377S. doi:10.3945/an.112.003533. ISSN 2161-8313. PMC 3650509. PMID 23674806.
- ^ Kovesdy, Csaba. "Pathogenesis, consequences, and treatment of metabolic acidosis in chronic kidney disease". UpToDate.
- ^ Stern, Scott D. C.; Cifu, Adam S.; Altkorn, Diane (2015). Symptom to diagnosis: an evidence-based guide (3rd ed.). New York: McGraw-Hill Education. ISBN 9780071803441. OCLC 896866189.
- ^ Quinn, Gene R.; Gleason, Nathaniel W.; Papadakis, Maxine A.; McPhee, Stephen J., eds. (2016). Current medical diagnosis & treatment study guide (2nd ed.). New York: McGraw-Hill. ISBN 9780071848053. OCLC 910475681.
- ^ a b DeGowin's diagnostic examination. LeBlond, Richard F.,, Brown, Donald D., 1940-, Suneja, Manish,, Szot, Joseph F. (Tenth ed.). New York. 2014-09-05. ISBN 9780071814478. OCLC 876336892.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ Morgan & Mikhail's clinical anesthesiology. Butterworth, John F., IV,, Mackey, David C.,, Wasnick, John D.,, Morgan, G. Edward,, Mikhail, Maged S.,, Morgan, G. Edward. (Sixth ed.). New York. 2018-08-21. ISBN 9781259834424. OCLC 1039081701.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ Smollin, Craig (2022). "Chapter 2-1: Acetaminophen". In Olson, Kent R.; Smollin, Craig G.; Anderson, Ilene B.; Benowitz, Neal L.; Blanc, Paul D.; Kim-Katz, Susan Y.; Lewis, Justin C.; Wu, Alan H.B. (eds.). Poisoning & Drug Overdose. The Faculty, Staff, and Associates of the California Poison Control System (Eighth ed.). McGraw Hill Lange. ISBN 978-1-264-25908-3.
- ^ Critical care. Oropello, John M.,, Pastores, Stephen M.,, Kvetan, Vladimir. [New York]. 2016-11-22. ISBN 9780071817264. OCLC 961480454.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ Current medical diagnosis & treatment 2020. Papadakis, Maxine A.,, McPhee, Stephen J.,, Rabow, Michael W. (Fifty-eighth ed.). New York. 2019-09-02. ISBN 9781260455281. OCLC 1109935506.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ Harrison's principles of internal medicine. Jameson, J. Larry,, Kasper, Dennis L.,, Longo, Dan L. (Dan Louis), 1949-, Fauci, Anthony S., 1940-, Hauser, Stephen L.,, Loscalzo, Joseph (20th ed.). New York. 2018-08-13. ISBN 9781259644030. OCLC 1029074059.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ Morgan & Mikhail's clinical anesthesiology. Butterworth, John F., IV,, Mackey, David C.,, Wasnick, John D.,, Morgan, G. Edward,, Mikhail, Maged S.,, Morgan, G. Edward. (Sixth ed.). New York. 2018-08-21. ISBN 978-1259834424. OCLC 1039081701.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ Katzung, Bertram G. (2018-09-05). Katzung & Trevor's pharmacology : examination & board review. Kruidering-Hall, Marieke,, Trevor, Anthony J. (Twelfth ed.). New York. ISBN 978-1259641022. OCLC 1052466341.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Levitzky, Michael G. (2007). Pulmonary physiology (7th ed.). New York: McGraw-Hill Medical. ISBN 9780071437752. OCLC 75713147.
- ^ Field, Michael J.; Pollock, Carol A.; Harris, David C. (2010). The renal system: basic science and clinical conditions (2nd ed.). Edinburgh: Churchill Livingstone/Elsevier. ISBN 9780702033711. OCLC 319855752.
- ^ Costanzo, Linda S. (2017-03-15). Physiology (6th ed.). Philadelphia, PA: Elsevier. ISBN 9780323511896. OCLC 965761862.
- ^ a b Kraut, Jeffrey A.; Madias, Nicolaos E. (2017). "Adverse Effects of the Metabolic Acidosis of Chronic Kidney Disease". Advances in Chronic Kidney Disease. 24 (5): 289–297. doi:10.1053/j.ackd.2017.06.005. PMID 29031355.
- ^ Kato, Akihiko; Kido, Ryo; Onishi, Yoshihiro; Kurita, Noriaki; Fukagawa, Masafumi; Akizawa, Tadao; Fukuhara, Shunichi (2014). "Association of serum bicarbonate with bone fractures in hemodialysis patients: the mineral and bone disorder outcomes study for Japanese CKD stage 5D patients (MBD-5D)". Nephron Clinical Practice. 128 (1–2): 79–87. doi:10.1159/000365089. ISSN 1660-2110. PMID 25378374. S2CID 20320396.
- ^ Lefebvre, A.; de Vernejoul, M. C.; Gueris, J.; Goldfarb, B.; Graulet, A. M.; Morieux, C. (1989). "Optimal correction of acidosis changes progression of dialysis osteodystrophy". Kidney International. 36 (6): 1112–1118. doi:10.1038/ki.1989.309. ISSN 0085-2538. PMID 2557481.
- ^ Hanna, Ramy M.; Ghobry, Lena; Wassef, Olivia; Rhee, Connie M.; Kalantar-Zadeh, Kamyar (2020). "A Practical Approach to Nutrition, Protein-Energy Wasting, Sarcopenia, and Cachexia in Patients with Chronic Kidney Disease". Blood Purification. 49 (1–2): 202–211. doi:10.1159/000504240. ISSN 0253-5068. PMID 31851983. S2CID 209418220.
- ^ Foley, Robert N.; Wang, Changchun; Ishani, Areef; Collins, Allan J.; Murray, Anne M. (2007). "Kidney Function and Sarcopenia in the United States General Population: NHANES III". American Journal of Nephrology. 27 (3): 279–286. doi:10.1159/000101827. ISSN 0250-8095. PMID 17440263. S2CID 2847009.
- ^ Shah, Samir N.; Abramowitz, Matthew; Hostetter, Thomas H.; Melamed, Michal L. (2009-08-01). "Serum bicarbonate levels and the progression of kidney disease: a cohort study". American Journal of Kidney Diseases. 54 (2): 270–277. doi:10.1053/j.ajkd.2009.02.014. ISSN 1523-6838. PMC 4354889. PMID 19394734.
- ^ Dobre, Mirela; Yang, Wei; Chen, Jing; Drawz, Paul; Hamm, L. Lee; Horwitz, Edward; Hostetter, Thomas; Jaar, Bernard; Lora, Claudia M.; Nessel, Lisa; Ojo, Akinlolu (2013-10-01). "Association of Serum Bicarbonate With Risk of Renal and Cardiovascular Outcomes in CKD: A Report From the Chronic Renal Insufficiency Cohort (CRIC) Study". American Journal of Kidney Diseases. 62 (4): 670–678. doi:10.1053/j.ajkd.2013.01.017. ISSN 0272-6386. PMC 3701754. PMID 23489677.
- ^ Menon, Vandana; Tighiouart, Hocine; Vaughn, Nubia Smith; Beck, Gerald J.; Kusek, John W.; Collins, Allan J.; Greene, Tom; Sarnak, Mark J. (2010-11-01). "Serum Bicarbonate and Long-term Outcomes in CKD". American Journal of Kidney Diseases. 56 (5): 907–914. doi:10.1053/j.ajkd.2010.03.023. ISSN 0272-6386. PMID 20605301.
- ^ Raphael, Kalani L.; Wei, Guo; Baird, Bradley C.; Greene, Tom; Beddhu, Srinivasan (2011-02-01). "Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans". Kidney International. 79 (3): 356–362. doi:10.1038/ki.2010.388. ISSN 0085-2538. PMC 5241271. PMID 20962743.
- ^ Jaber, Samir; Paugam, Catherine; Futier, Emmanuel; et al. (2018). "Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial" (PDF). The Lancet. 392 (10141): 31–40. doi:10.1016/S0140-6736(18)31080-8. PMID 29910040. S2CID 49276138.
- ^ Goraya, Nimrit; Wesson, Donald E. (2019). "Clinical evidence that treatment of metabolic acidosis slows the progression of chronic kidney disease". Current Opinion in Nephrology and Hypertension. 28 (3): 267–277. doi:10.1097/MNH.0000000000000491. ISSN 1062-4821. PMC 6467553. PMID 30681417.
- ^ Garneata, Liliana; Stancu, Alexandra; Dragomir, Diana; Stefan, Gabriel; Mircescu, Gabriel (2016-07-01). "Ketoanalogue-Supplemented Vegetarian Very Low–Protein Diet and CKD Progression". Journal of the American Society of Nephrology. 27 (7): 2164–2176. doi:10.1681/ASN.2015040369. ISSN 1046-6673. PMC 4926970. PMID 26823552.
- ^ Chen, Wei; Abramowitz, Matthew K. (2019). "Advances in management of chronic metabolic acidosis in chronic kidney disease". Current Opinion in Nephrology and Hypertension. 28 (5): 409–416. doi:10.1097/MNH.0000000000000524. ISSN 1473-6543. PMC 6677263. PMID 31232712.
- ^ Wesson, Donald E.; Mathur, Vandana; Tangri, Navdeep; Stasiv, Yuri; Parsell, Dawn; Li, Elizabeth; Klaerner, Gerrit; Bushinsky, David A. (2019-04-06). "Veverimer versus placebo in patients with metabolic acidosis associated with chronic kidney disease: a multicentre, randomised, double-blind, controlled, phase 3 trial". The Lancet. 393 (10179): 1417–1427. doi:10.1016/S0140-6736(18)32562-5. ISSN 0140-6736. PMID 30857647. S2CID 72332908.
- ^ Wesson, Donald E.; Mathur, Vandana; Tangri, Navdeep; Stasiv, Yuri; Parsell, Dawn; Li, Elizabeth; Klaerner, Gerrit; Bushinsky, David A. (2019-08-03). "Long-term safety and efficacy of veverimer in patients with metabolic acidosis in chronic kidney disease: a multicentre, randomised, blinded, placebo-controlled, 40-week extension". The Lancet. 394 (10196): 396–406. doi:10.1016/S0140-6736(19)31388-1. ISSN 0140-6736. PMID 31248662. S2CID 195339720.