Etravirine (ETR,[3]), sold under the brand name Intelence is an antiretroviral medication used for the treatment of HIV.[1] Etravirine is a human immunodeficiency virus type 1 (HIV-1) non-nucleoside reverse transcriptase inhibitor (NNRTI).[1] Unlike agents in the class, resistance to other NNRTIs does not seem to confer resistance to etravirine.[4] Etravirine is marketed by Janssen, a subsidiary of Johnson & Johnson. In January 2008, the US Food and Drug Administration (FDA) approved its use for people with established resistance to other drugs, making it the 30th anti-HIV drug approved in the United States and the first to be approved in 2008.[5] It was also approved for use in Canada in April 2008.[6]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Intelence |
| Other names | TMC125 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608016 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 99.9% |
| Metabolism | Liver (CYP3A4, CYP2C9 & CYP2C19-mediated) |
| Elimination half-life | 41±20 hours |
| Excretion | Faeces (93.7%), urine (1.2%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.207.546 |
| Chemical and physical data | |
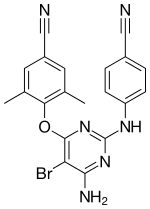
| Formula | C20H15BrN6O |
| Molar mass | 435.285 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Etravirine is licensed in the United States, Canada, Israel, Russia, Australia, New Zealand, and the European Union,[7] and is under regulatory review in Switzerland.[8]
Medical uses
editIn the US, etravirine is indicated for the treatment of HIV-1 infection in treatment-experienced patients aged two years of age and older.[1]
In the EU, etravirine, in combination with a boosted protease inhibitor and other antiretrovirals, is indicated for the treatment of human-immunodeficiency-virus-type-1 (HIV-1) infection in antiretroviral-treatment-experienced people aged six years of age and older.[2]
Contraindication
editPeople with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this etravine.[9]
Adverse effects
editIn 2009, the FDA prescribing information for etravirine was modified to include "postmarketing reports of cases of Stevens–Johnson syndrome, toxic epidermal necrolysis, and erythema multiforme, as well as hypersensitivity reactions characterized by rash, constitutional findings, and sometimes organ dysfunction, including liver failure."[10]
Mechanism of action
editEtravirine is a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI), designed to be active against HIV with mutations that confer resistance to the two most commonly prescribed first-generation NNRTIs, mutation K103N for efavirenz and Y181C for nevirapine.[11] This potency appears to be related to etravirine's flexibility as a molecule. Etravirine is a diarylpyrimidine (DAPY), a type of organic molecule with some conformational isomerism that can bind the enzyme reverse transcriptase in multiple conformations, allowing for a more robust interaction between etravirine and the enzyme, even in the presence of mutations.[12]
Chemistry
editEtravine forms as colourless orthorhombic crystals in space group Pna21.[13] The structures of these and of a number of solvate and salt forms have been reported.[13][14]
Research
editEtravine has been studied for use in a drug repositioning application. Etravirine was shown to cause an increase in frataxin production.[15]
References
edit- ^ a b c d "Intelence- etravirine tablet". DailyMed. 15 August 2023. Retrieved 14 August 2024.
- ^ a b "Intelence EPAR". European Medicines Agency (EMA). 28 August 2008. Retrieved 14 August 2024. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Appendix A: Key to Acronyms". Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services. Archived from the original on 31 August 2012.
- ^ Stellbrink HJ (October 2007). "Antiviral drugs in the treatment of AIDS: what is in the pipeline ?". European Journal of Medical Research. 12 (9): 483–495. PMID 17933730.
- ^ "FDA Approves HIV Drug Etravirine". Associated Press. 18 January 2008.[dead link]
- ^ "First New NNRTI in Nearly a Decade to Benefit Canadians with HIV/AIDS" (PDF) (Press release). Janssen-Ortho Inc. 1 April 2008. Archived from the original (PDF) on 2 November 2010. Retrieved 9 July 2008.
- ^ "Intelence receives marketing authorisation in the European Union for HIV combination therapy". Tibotec. Archived from the original on 28 September 2011. Retrieved 29 August 2008.
- ^ "Etravirine (TMC125, Intelence) granted accelerated approval in US". aidsmap. Archived from the original on 2 January 2010. Retrieved 24 January 2008.
- ^ "Etravine: Summary of product characteristics" (PDF). EMEA. p. 5. Archived from the original (PDF) on 20 August 2016. Retrieved 13 July 2011.
- ^ "FDA Medwatch Safety Information". Food and Drug Administration. Retrieved 27 August 2009.[dead link]
- ^ Evans D (15 January 2008). "Etravirine—Countdown to Launch". AIDSmeds.com. Archived from the original on 19 January 2008. Retrieved 2 February 2008.
- ^ Das K, Clark AD, Lewi PJ, Heeres J, De Jonge MR, Koymans LM, et al. (May 2004). "Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants". Journal of Medicinal Chemistry. 47 (10): 2550–2560. doi:10.1021/jm030558s. PMID 15115397.
- ^ a b Rajput L, Sanphui P, Desiraju GR (7 August 2013). "New Solid Forms of the Anti-HIV Drug Etravirine: Salts, Cocrystals, and Solubility". Crystal Growth & Design. 13 (8): 3681–3690. doi:10.1021/cg4007058. ISSN 1528-7483.
- ^ Muresan-Pop M, Macavei S, Turza A, Borodi G (November 2021). "New solvates and a salt of the anti-HIV compound etravirine". Acta Crystallographica Section C: Structural Chemistry. 77 (Pt 11): 698–706. doi:10.1107/S2053229621010482. PMID 34738540. S2CID 243761396.
- ^ Alfedi G, Luffarelli R, Condò I, Pedini G, Mannucci L, Massaro DS, et al. (March 2019). "Drug repositioning screening identifies etravirine as a potential therapeutic for friedreich's ataxia". Movement Disorders. 34 (3): 323–334. doi:10.1002/mds.27604. PMID 30624801. S2CID 58567610.