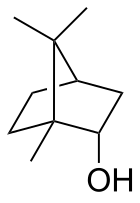
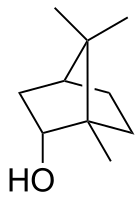
Borneol is a bicyclic organic compound and a terpene derivative. The hydroxyl group in this compound is placed in an endo position. The exo diastereomer is called isoborneol. Being chiral, borneol exists as enantiomers, both of which are found in nature: d-borneol (also written (+)-borneol) and l-borneol ((-)-borneol).
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
rel-(1R,2S,4R)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ol
| |||
| Other names
1,7,7-Trimethylbicyclo[2.2.1]heptan-2-endo-ol
endo-2-Bornanol, Borneo camphor | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.006.685 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| UN number | 1312 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H18O | |||
| Molar mass | 154.253 g·mol−1 | ||
| Appearance | colorless to white lumps | ||
| Odor | pungent, camphor-like | ||
| Density | 1.011 g/cm3 (20 °C)[1] | ||
| Melting point | 208 °C (406 °F; 481 K) | ||
| Boiling point | 213 °C (415 °F; 486 K) | ||
| slightly soluble (D-form) | |||
| Solubility | soluble in chloroform, ethanol, acetone, ether, benzene, toluene, decalin, tetralin | ||
| −1.26×10−4 cm3/mol | |||
| Hazards | |||
| GHS labelling: | |||

| |||
| Warning | |||
| H228 | |||
| P210, P240, P241, P280, P370+P378 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 65 °C (149 °F; 338 K) | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Related compounds
|
Bornane (hydrocarbon) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Reactions
editOccurrence
editThe compound was named in 1842 by the French chemist Charles Frédéric Gerhardt.[2] Borneol can be found in several species of Heterotheca,[3] Artemisia, Rosmarinus officinalis (rosemary)[4] Dipterocarpaceae, Blumea balsamifera and Kaempferia galanga.[5]
It is one of the chemical compounds found in castoreum. This compound is gathered from the beaver's plant food.[6]
Synthesis
editBorneol can be synthesized by reduction of camphor by the Meerwein–Ponndorf–Verley reduction (a reversible process). Industrially, a racemic mixture of camphor is used, leading to a racemic mixture of borneol and isoborneol. The chirality can be controlled by changing the chirality of camphor: (+)-camphor gives (−)-isoborneol and (+)-borneol.[7]
Reduction of camphor with sodium borohydride (fast and irreversible) gives instead the diastereomer isoborneol.
Natural sources
editIndustrially, natural (+)-borneol is produced from Cinnamomum burmanni (one specific chemotype)[8] and Cinnamomum camphora.[9]
Natural (-)-borneol occurs in Blumea balsamifera.
Biosynthesis
editBorneol is synthesized using DMAPP as the starting material. DMAPP is then converted to GPP, which is acted upon by a bornyl diphosphate synthase to yield a bornyl diphosphate. A phosphatase then removes the phosphate groups, yielding borneol.[10]
The chirality of borneol in a plant depends on the preferred chirality of the bornyl diphosphate synthase. Synthases for either chirality have been sequenced.[10][11]
A downstream product is camphor of either chirality, a reaction catalyzed by (+)-borneol dehydrogenase or (-)-borneol dehydrogenase.
Uses
editWhereas d-borneol was the enantiomer that used to be the most readily available commercially, the more commercially available enantiomer now is l-borneol, which also occurs in nature.
(+)-Borneol from Dipterocarpus spp. is used in traditional Chinese medicine. An early description is found in the Bencao Gangmu.
Borneol is a component of many essential oils[12] and it is a natural insect repellent.[13] It also generates a TRPM8-mediated cooling sensation similar to menthol.[14]
Laevo-borneol is used in perfumery. It has a balsamic odour type with pine, woody and camphoraceous facets.
Dextro-borneol (dexborneol) is used in edaravone/dexborneol, a drug approved in China for stroke.[15]
Toxicology
editBorneol may cause eye, skin, and respiratory irritation; it is harmful if swallowed.[16] Acute exposure may cause headache, nausea, vomiting, dizziness, lightheadedness, and syncope. Exposure to higher levels or over a longer period of time may cause restlessness, difficulty concentrating, irritability, and seizures.[17]
Skin irritation
editBorneol has been shown to have little to no irritation effect when applied to the human skin at doses used in fine fragrance formulation.[18] Skin exposure can lead to sensitization and a future allergic reaction even to small quantities.[17]
Derivatives
editThe bornyl group is a univalent radical C10H17 derived from borneol by removal of hydroxyl and is also known as 2-bornyl.[19] Isobornyl is the univalent radical C10H17 that is derived from isoborneol.[20] The structural isomer fenchol is also a widely used compound derived from certain essential oils.
Bornyl acetate is the acetate ester of borneol.
Notes and references
edit- ^ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton, Florida: CRC Press. p. 3.56. ISBN 0-8493-0486-5.
- ^ C. Gerhardt (1842) "Sur la transformation de l'essence de valériane en camphre de Bornéo et en camphre des laurinées" (On the transformation of the essence of valerian into Borneo camphor and into laurel camphor), Comptes rendus, 14 : 832-835. From p. 834: "Je donne, par cette raison, à l'hydrogène carboné de l'essence de valériane, le nom de bornéène, et, au camphre lui-même, celui de bornéol." (I give, for this reason [namely, that the compound that Gerhardt had obtained from valerian oil was identical to that obtained by Pelouze from camphor from Borneo], to the hydrocarbon from valerian essence, the name bornéène, and, to camphor itself, that of borneol.)
- ^ Lincoln, D.E., B.M. Lawrence. 1984. "The volatile constituents of camphorweed, Heterotheca subaxillaris". Phytochemistry 23(4): 933-934
- ^ Begum, A.; Sandhya, S.; Shaffath Ali, S.; Vinod, K. R.; Reddy, S.; Banji, D. (2013). "An in-depth review on the medicinal flora Rosmarinus officinalis (Lamiaceae)". Acta Scientiarum Polonorum. Technologia Alimentaria. 12 (1): 61–73. PMID 24584866.
- ^ Wong, K. C.; Ong, K. S.; Lim, C. L. (2006). "Composition of the essential oil of rhizomes of Kaempferia Galanga L.". Flavour and Fragrance Journal. 7 (5): 263–266. doi:10.1002/ffj.2730070506.
- ^ The Beaver: Its Life and Impact. Dietland Muller-Schwarze, 2003, page 43 (book at google books)
- ^ Yang, Ming-Yeh; Khine, Aye Aye; Liu, Jen-Wei; Cheng, Hui-Chen; Hu, Anren; Chen, Hao-Ping; Shih, Tzenge-Lien (November 2018). "Resolution of isoborneol and its isomers by GC/MS to identify "synthetic" and "semi-synthetic" borneol products". Chirality. 30 (11): 1233–1239. doi:10.1002/chir.23017. PMID 30222211.
- ^ Li, Fangping; Huang, Shilin; Mei, Yu; Wu, Bingqi; Hou, Zhuangwei; Zhan, Penglin; Hou, Zhihao; Huang, Wenjie; Zhao, Junliang; Wang, Jihua (October 2022). "Genome assembly provided new insights into the Cinnamomum burmannii evolution and D-borneol biosynthesis differences between chemotypes". Industrial Crops and Products. 186: 115181. doi:10.1016/j.indcrop.2022.115181.
- ^ Xingxing, Liu; Xi, Zhang; Xiali, Guo; Shangji, Gong; Xiangmei, Jiang; Yuxin, Fu; Liping, Luo (2014). "Multivariate Analyses of Volatile Chemical Composition in Leaves of Different Cinnamomum camphora Chemotypes". Chinese Bulletin of Botany. 49 (2): 161. doi:10.3724/SP.J.1259.2014.00161.
- ^ a b Ma, Rui; Su, Ping; Ma, Qing; Guo, Juan; Chen, Suiqing; Jin, Baolong; Zhang, Haiyan; Tang, Jinfu; Zhou, Tao; Xiao, Chenghong; Cui, Guanghong; Huang, Luqi (March 2022). "Identification of (-)-bornyl diphosphate synthase from Blumea balsamifera and its application for (-)-borneol biosynthesis in Saccharomyces cerevisiae". Synthetic and Systems Biotechnology. 7 (1): 490–497. doi:10.1016/j.synbio.2021.12.004. PMC 8671873. PMID 34977393.
- ^ Yang, Zerui; An, Wenli; Liu, Shanshan; Huang, Yuying; Xie, Chunzhu; Huang, Song; Zheng, Xiasheng (10 June 2020). "Mining of candidate genes involved in the biosynthesis of dextrorotatory borneol in Cinnamomum burmannii by transcriptomic analysis on three chemotypes". PeerJ. 8: e9311. doi:10.7717/peerj.9311. PMC 7293187. PMID 32566406.
- ^ Plants containing borneol Archived 2015-09-23 at the Wayback Machine (Dr. Duke's Phytochemical and Ethnobotanical Databases)]
- ^ "Chemical Information". sun.ars-grin.gov. Archived from the original on 2004-11-07. Retrieved 2008-03-02.
- ^ Chen, GL; Lei, M; Zhou, LP; Zeng, B; Zou, F (2016). "Borneol Is a TRPM8 Agonist that Increases Ocular Surface Wetness". PLOS ONE. 11 (7): e0158868. Bibcode:2016PLoSO..1158868C. doi:10.1371/journal.pone.0158868. PMC 4957794. PMID 27448228.
- ^ Xu, Jie; Wang, Anxin; Meng, Xia; Yalkun, Gulbahram; Xu, Anding; Gao, Zhiqiang; Chen, Huisheng; Ji, Yong; Xu, Jun; Geng, Deqin; Zhu, Runxiu; Liu, Bo; Dong, Aiqin; Mu, Hua; Lu, Zhihong; Li, Shuya; Zheng, Huaguang; Chen, Xia; Wang, Yilong; Zhao, Xingquan; Wang, Yongjun; Wang, Yongjun; Xu, Anding; Zhao, Xingquan; Chen, Xia; Wang, Yongjun; Meng, Xia; Wang, Yilong; Xu, Jie; Wang, Anxin; Zheng, Huaguang; Gao, Zhiqiang; Duan, Lei; Zhang, Jinghua; Li, Shuya; Lou, Donghua; Gao, Zhiqiang; Chen, Huisheng; Ji, Yong; Xu, Jun; Geng, Deqin; Zhu, Runxiu; Liu, Bo; Dong, Aiqin; Liang, Qingcheng; Yang, Hong; Guo, Cunju; Li, Xin; He, Mingli; Tian, Xiangyang; Cui, Yong; Zhou, Junshan; Wang, Ning; Wang, Lei; Zhang, Xinjiang; Gao, Xiaoping; Lu, Liping; Li, Tong; Cheng, Yan; Liu, Kaixiang; Xi, Xiaokun; Wang, Baojun; Sun, Lin; Zhao, Shigang; Chu, Xiaofan; Lian, Yajun; Yan, Fuling; Wang, Xiaoshan; Wang, Dong; Shao, Bei; Jiao, Jinsong; Wu, Heng; Li, Guanglai; Guo, Libin; Wang, Yongjun; Pan, Suyue; Xu, Anding; Li, Heng; Zhuang, Jianhua; Li, Xin; Wu, Jun; Wang, Anxin; Lou, Donghua; Zuo, Yingting; Zhang, Yijun; Zhang, Xiaoli; Feng, Xiaofei; Meng, Xia; Wang, David; Dong, Kehui; Liu, Yanfang; Li, Hao; Chen, Dawei; Lv, Qiushi (March 2021). "Edaravone Dexborneol Versus Edaravone Alone for the Treatment of Acute Ischemic Stroke: A Phase III, Randomized, Double-Blind, Comparative Trial". Stroke. 52 (3): 772–780. doi:10.1161/STROKEAHA.120.031197. PMID 33588596.
- ^ Material Safety Data Sheet. Fisher Scientific.
- ^ a b HAZARDOUS SUBSTANCE FACT SHEET (PDF)
- ^ Bhatia, S.P.; Letizia, C.S.; Api, A.M. (November 2008). "Fragrance material review on borneol". Food and Chemical Toxicology. 46 (11): S77–S80. doi:10.1016/j.fct.2008.06.031. PMID 18640181.
- ^ "Definition of BORNYL". www.merriam-webster.com.
- ^ "Definition of ISOBORNYL". www.merriam-webster.com.