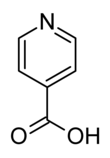
Isonicotinic acid or pyridine-4-carboxylic acid is an organic compound with the formula C5H4N(CO2H). It is a derivative of pyridine with a carboxylic acid substituent at the 4-position. It is an isomer of picolinic acid and nicotinic acid, which have the carboxyl group at the 2- and 3-position respectively compared to the 4-position for isonicotinic acid.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Pyridine-4-carboxylic acid | |||
| Other names
Isonicotinic acid
4-Pyridinecarboxylic acid p-Pyridinecarboxylic acid 4-Picolinic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.208 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H5NO2 | |||
| Molar mass | 123.111 g·mol−1 | ||
| Appearance | White to off-white crystalline solid | ||
| Density | Solid | ||
| Melting point | 310 °C (590 °F; 583 K) (sublimes) | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Safety data sheet (SDS) | http://datasheets.scbt.com/sc-250188.pdf | ||
| Related compounds | |||
Related compounds
|
nicotinic acid, pyridine isoniazid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Production
editOn a commercial scale, isonicotinic acid, like other pyridine carboxylic acid is produced by ammoxidation of 4-picoline (4-methylpyridine) followed by hydrolysis of the resulting nitrile:
- NC5H4CH3 + 1.5 O2 + NH3 → NC5H4C≡N + 3 H2O
- NC5H4C≡N + 2 H2O → NC5H4CO2H + NH3
It is also produced by oxidation of 4-picoline with nitric acid.[2]
Derivatives
editIsonicotinic acids is a term loosely used for derivatives of isonicotinic acid. Hydrazide derivatives include isoniazid, iproniazid, and nialamide. Amide and ester derivatives include ethionamide and dexamethasone isonicotinate.
Its conjugate base forms coordination polymers[3] and MOFs[4] by binding metal ions through both the N and carboxylate.
See also
editReferences
edit- ^ Isonicotinic acid at chemicalland21.com
- ^ Shimizu, Shinkichi; Watanabe, Nanao; Kataoka, Toshiaki; Shoji, Takayuki; Abe, Nobuyuki; Morishita, Sinji; Ichimura, Hisao (2007). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_399. ISBN 978-3527306732.
- ^ Huang, Wei; Zhu, Hai-Bin; Gou, Shao-Hua (2006). "Self-assembly directed by dinuclear zinc(II) macrocyclic species". Coordination Chemistry Reviews. 250 (3–4): 414–423. doi:10.1016/j.ccr.2005.07.008.
- ^ Lin, Rui-Biao; Wu, Hui; Li, Libo; Tang, Xiao-Liang; Li, Zhiqiang; Gao, Junkuo; Cui, Hui; Zhou, Wei; Chen, Banglin (2018). "Boosting Ethane/Ethylene Separation within Isoreticular Ultramicroporous Metal–Organic Frameworks". Journal of the American Chemical Society. 140 (40): 12940–12946. doi:10.1021/jacs.8b07563. PMID 30216725.
External links
edit- Isonicotinic+Acids at the U.S. National Library of Medicine Medical Subject Headings (MeSH)