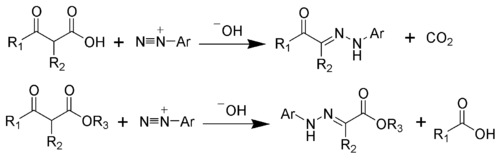
The Japp–Klingemann reaction is a chemical reaction used to synthesize hydrazones from β-keto-acids (or β-keto-esters) and aryl diazonium salts.[1][2][3][4][5][6] The reaction is named after the chemists Francis Robert Japp and Felix Klingemann.
| Japp–Klingemann reaction | |
|---|---|
| Named after | Francis Robert Japp Felix Klingemann |
| Reaction type | Coupling reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000158 |
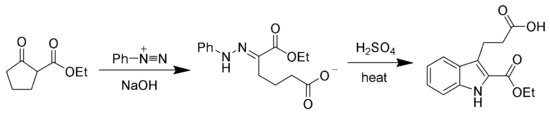
The hydrazone products of the Japp–Klingemann reaction are most often used as intermediates in syntheses of more complex organic molecules. For example, a phenylhydrazone product can be heated in the presence of strong acid to produce an indole via the Fischer indole synthesis.[7][8]
If there is a leaving group elsewhere in the Japp–Klingemann product, the hydrazone instead can cyclize at that site via a substitution reaction to give a pyrazole. This process is a key part of the synthesis of pyraclofos and related compounds:[9]
Reaction mechanism
editTo illustrate the mechanism, the Japp-Klingemann ester variation will be considered. The first step is the deprotonation of the β-keto-ester. The nucleophilic addition of the enolate anion 2 to the diazonium salt produces the azo compound 3. Intermediate 3 has been isolated in rare cases. However, in most cases, the hydrolysis of intermediate 3 produces a tetrahedral intermediate 4, which quickly decomposes to release the carboxylic acid 6. After hydrogen exchange, the final hydrazone 7 is produced.
References
edit- ^ Francis Robert Japp, Felix Klingemann (1887). "Ueber Benzolazo- und Benzolhydrazofettsäuren". Berichte der deutschen chemischen Gesellschaft. 20 (2): 2942–2944. doi:10.1002/cber.188702002165.
- ^ F. R. Japp; F. Klingemann (1887). "Zur Kenntniss der Benzolazo- und Benzolhydrazopropionsäuren (p 3284-3286)". Berichte der Deutschen Chemischen Gesellschaft. 20 (2): 3284–3286. doi:10.1002/cber.188702002234.
- ^ F. R. Japp; F. Klingemann (1887). "Ueber sogenannte »gemischte Azoverbindungen". Berichte der deutschen chemischen Gesellschaft. 20 (2): 3398–3401. doi:10.1002/cber.188702002268.
- ^ F. R. Japp; F. Klingemann (1888). "Ueber die Constitution einiger sogenannten gemischten Azoverbindungen". Liebigs Annalen der Chemie. 247 (2): 190–225. doi:10.1002/jlac.18882470208.
- ^ Phillips, R. R. Org. React. 1959, 10, 143.
- ^ Reynolds, G. A.; VanAllan, J. A. Org. Synth., Coll. Vol. 4, p.633 (1963); Vol. 32, p.84 (1952)(Article Archived 2012-07-16 at the Wayback Machine)
- ^ Bowman, R. E.; Goodburn, T. G.; Reynolds, A. A. (1972). "1,3,4,5-Tetrahydrobenz[cd]indoles and related compounds. Part I. A new synthesis of 3,4-dihydrobenz[cd]indol-5(1H)-one (Uhle's ketone)". J. Chem. Soc. Perkin Trans. 1: 1121. doi:10.1039/P19720001121.
- ^ Meyer, M. D.; Kruse, L. I. (1984). "Ergoline synthons: Synthesis of 3,4-dihydro-6-methoxybenz[cd]indol-5(1H)-one (6-methoxy-Uhle's ketone) and 3,4-dihydrobenz[cd]indol-5(1H)-one (Uhle's ketone) via a novel decarboxylation of indole-2-carboxylates". J. Org. Chem. 49 (17): 3195–3199. doi:10.1021/jo00191a028.
- ^ Lamberth, Clemens (2002). "An improved procedure for the preparation of 1-aryl-4-hydroxy-1H-pyrazoles". Organic Preprarations and Procedures International. 34 (1): 98–102. doi:10.1080/00304940209355748.