Chlorpromazine (CPZ), marketed under the brand names Thorazine and Largactil among others, is an antipsychotic medication.[6] It is primarily used to treat psychotic disorders such as schizophrenia.[6] Other uses include the treatment of bipolar disorder, severe behavioral problems in children including those with attention deficit hyperactivity disorder, nausea and vomiting, anxiety before surgery, and hiccups that do not improve following other measures.[6] It can be given orally (by mouth), by intramuscular injection (injection into a muscle), or intravenously (injection into a vein).[6]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Largactil, Thorazine, Sonazine, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682040 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, rectal, intramuscular, intravenous |
| Drug class | Typical antipsychotic |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 10–80% (Oral; large interindividual variation)[5] |
| Protein binding | 90–99%[5] |
| Metabolism | Liver, mostly CYP2D6-mediated[5] |
| Elimination half-life | 30 hours[6] |
| Excretion | Kidney (43–65% in 24 hrs)[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.042 |
| Chemical and physical data | |
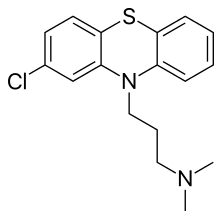
| Formula | C17H19ClN2S |
| Molar mass | 318.86 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Chlorpromazine is in the typical antipsychotic class,[6] and, chemically, is one of the phenothiazines. Its mechanism of action is not entirely clear but is believed to be related to its ability as a dopamine antagonist.[6] It has antiserotonergic and antihistaminergic properties.[6]
Common side effects include movement problems, sleepiness, dry mouth, low blood pressure upon standing, and increased weight.[6] Serious side effects may include the potentially permanent movement disorder tardive dyskinesia, neuroleptic malignant syndrome, severe lowering of the seizure threshold, and low white blood cell levels.[6] In older people with psychosis as a result of dementia, it may increase the risk of death.[6] It is unclear if it is safe for use in pregnancy.[6]
Chlorpromazine was developed in 1950 and was the first antipsychotic on the market.[7][8] It is on the World Health Organization's List of Essential Medicines.[9][10] Its introduction has been labeled as one of the great advances in the history of psychiatry.[11][12] It is available as a generic medication.[6]
Medical uses
editChlorpromazine is used in the treatment of both acute and chronic psychoses, including schizophrenia and the manic phase of bipolar disorder, as well as amphetamine-induced psychosis.
Controversially, some psychiatric ward patients may be given Chlorpromazine by force, even if they do not suffer any of the typical conditions the drug is prescribed for.[13]
In a 2013 comparison of fifteen antipsychotics in schizophrenia, chlorpromazine demonstrated mild-standard effectiveness. It was 13% more effective than lurasidone and iloperidone, approximately as effective as ziprasidone and asenapine, and 12–16% less effective than haloperidol, quetiapine, and aripiprazole.[14]
A 2014 systematic review carried out by Cochrane included 55 trials that compared the effectiveness of chlorpromazine versus placebo for the treatment of schizophrenia. Compared to the placebo group, patients under chlorpromazine experienced less relapse during 6 months to 2 years follow-up. No difference was found between the two groups beyond two years of follow-up. Patients under chlorpromazine showed a global improvement in symptoms and functioning. The systematic review also highlighted the fact that the side effects of the drug were 'severe and debilitating', including sedation, considerable weight gain, a lowering of blood pressure, and an increased risk of acute movement disorders. They also noted that the quality of evidence of the 55 included trials was very low and that 315 trials could not be included in the systematic review due to their poor quality. They called for further research on the subject, as chlorpromazine is a cheap benchmark drug and one of the most used treatments for schizophrenia worldwide.[15]
Chlorpromazine has also been used in porphyria and as part of tetanus treatment. It still is recommended for short-term management of severe anxiety and psychotic aggression. Resistant and severe hiccups, severe nausea/emesis, and preanesthetic conditioning are other uses.[16][17] Symptoms of delirium in hospitalized AIDS patients have been effectively treated with low doses of chlorpromazine.[18]
Other uses
editChlorpromazine is occasionally used off-label for treatment of severe migraine.[19][20] It is often, particularly as palliation, used in small doses to reduce nausea by opioid-treated cancer patients and to intensify and prolong the analgesia of the opioids as well.[19][21] Efficacy has been shown in treatment of symptomatic hypertensive emergency.
In Germany, chlorpromazine still carries label indications for insomnia, severe pruritus, and preanesthesia.[22]
Chlorpromazine has been used as a hallucinogen antidote or "trip killer" to block the effects of serotonergic psychedelics like psilocybin, lysergic acid diethylamide (LSD), and mescaline.[23][24][25] However, the results of clinical studies of chlorpromazine for this use have been inconsistent, with reduced effects, no change in effects, and even enhanced effects all reported.[23]
Chlorpromazine and other phenothiazines have been demonstrated to possess antimicrobial properties, but are not currently used for this purpose except for a very small number of cases. For example, Miki et al. 1992 trialed daily doses of chlorpromazine, reversing chloroquine resistance in Plasmodium chabaudi isolates in mice.[26] Weeks et al., 2018 find that it also possesses a wide spectrum anthelmintic effect.[27]
Chlorpromazine is an antagonist of several insect monoamine receptors.[28] It is the most active antagonist known of silk moth (Bombyx mori) octopamine receptor α, intermediate for Bm tyramine receptors 1 & 2, weak for Drosophila octopamine receptor β, high for Drosophila tyramine receptor 1, intermediate for migratory locust (Locusta migratoria) tyramine receptor 1, and high for American cockroach (Periplaneta americana) octopamine receptor α and tyramine receptor 1.[28]
| Measured outcome | Findings summary | Findings range | Quality of evidence |
|---|---|---|---|
| Global effects | |||
| No improvement (9 weeks – 6 months) | 30% less risk of having no improvement in mental state, behaviour and functioning | RR 0.7 CI 0.6 to 0.9 | Very low (estimate of effect uncertain) |
| Relapse (6 months – 2 years) | 35% less risk of relapse | RR 0.7 CI 0.5 to 0.9 | |
Adverse effects
editThere appears to be a dose-dependent risk for seizures with chlorpromazine treatment.[30] Tardive dyskinesia (involuntary, repetitive body movements) and akathisia (a feeling of inner restlessness and inability to stay still) are less commonly seen with chlorpromazine than they are with high potency typical antipsychotics such as haloperidol[31] or trifluoperazine, and some evidence suggests that, with conservative dosing, the incidence of such effects for chlorpromazine may be comparable to that of newer agents such as risperidone or olanzapine.[32]
Chlorpromazine may deposit in ocular tissues when taken in high dosages for long periods of time.
| Measured outcome | Findings summary | Findings range | Quality of evidence |
|---|---|---|---|
| Adverse effects | |||
| Weight gain | 5 times more likely to have considerable weight gain, around 40% with chlorpromazine gaining weight | RR 4.9 CI 2.3 to 10.4 | Very low (estimate of effect uncertain) |
| Sedation | 3 times more likely to cause sedation, around 30% with chlorpromazine | RR 2.8 CI 2.3 to 3.5 | |
| Acute movement disorder | 3.5 times more likely to cause easily reversible but unpleasant severe stiffening of muscles, around 6% with chlorpromazine | RR 3.5 CI 1.5 to 8.0 | |
| Parkinsonism | 2 times more likely to cause parkinsonism (symptoms such as tremor, hesitancy of movement, decreased facial expression), around 17% with chlorpromazine | RR 2.1 CI 1.6 to 2.8 | |
| Decreased blood pressure with dizziness | 3 times more likely to cause decreased blood pressure and dizziness, around 15% with chlorpromazine | RR 2.4 CI 1.7 to 3.3 | |
Contraindications
editAbsolute contraindications include:[5]
- Circulatory depression
- CNS depression
- Coma
- Drug intoxication
- Bone marrow suppression
- Phaeochromocytoma
- Hepatic failure
- Active liver disease
- Previous hypersensitivity (including jaundice, agranulocytosis, etc.) to phenothiazines, especially chlorpromazine, or any of the excipients in the formulation being used.
Relative contraindications include:[5]
- Epilepsy
- Parkinson's disease
- Myasthenia gravis
- Hypoparathyroidism
- Prostatic hypertrophy
Very rarely, elongation of the QT interval, due to hERG blockade, may occur, increasing the risk of potentially fatal arrhythmias.[33]
Interactions
editThis section may require cleanup to meet Wikipedia's quality standards. The specific problem is: Too much use of "also"; unclear structure/organization, especially due to heavy reliance on one source. (January 2022) |
Consuming food prior to taking chlorpromazine orally limits its absorption; likewise, cotreatment with benztropine can also reduce chlorpromazine absorption.[5] Alcohol can also reduce chlorpromazine absorption.[5] Antacids slow chlorpromazine absorption.[5] Lithium and chronic treatment with barbiturates can increase chlorpromazine clearance significantly.[5] Tricyclic antidepressants (TCAs) can decrease chlorpromazine clearance and hence increase chlorpromazine exposure.[5] Cotreatment with CYP1A2 inhibitors like ciprofloxacin, fluvoxamine or vemurafenib can reduce chlorpromazine clearance and hence increase exposure and potentially also adverse effects.[5] Chlorpromazine can also potentiate the CNS depressant effects of drugs like barbiturates, benzodiazepines, opioids, lithium and anesthetics and hence increase the potential for adverse effects such as respiratory depression and sedation.[5]
Chlorprozamine is also a moderate inhibitor of CYP2D6 and a substrate for CYP2D6, and hence can inhibit its own metabolism.[16] It can also inhibit the clearance of CYP2D6 substrates such as dextromethorphan, potentiating their effects.[16] Other drugs like codeine and tamoxifen, which require CYP2D6-mediated activation into their respective active metabolites, may have their therapeutic effects attenuated.[16] Likewise, CYP2D6 inhibitors such as paroxetine or fluoxetine can reduce chlorpromazine clearance, increasing serum levels of chlorpromazine and potentially its adverse effects.[5] Chlorpromazine also reduces phenytoin levels and increases valproic acid levels.[5] It also reduces propranolol clearance and antagonizes the therapeutic effects of antidiabetic agents, levodopa (a Parkinson's medication. This is likely due to the fact that chlorpromazine antagonizes the D2 receptor which is one of the receptors dopamine, a levodopa metabolite, activates), amphetamines and anticoagulants.[5] It may also interact with anticholinergic drugs such as orphenadrine to produce hypoglycaemia (low blood sugar).[5]
Chlorpromazine may also interact with epinephrine (adrenaline) to produce a paradoxical fall in blood pressure.[5] Monoamine oxidase inhibitors (MAOIs) and thiazide diuretics may also accentuate the orthostatic hypotension experienced by those receiving chlorpromazine treatment.[5] Quinidine may interact with chlorpromazine to increase myocardial depression.[5] Likewise, it may also antagonize the effects of clonidine and guanethidine.[5] It also may reduce the seizure threshold and hence a corresponding titration of anticonvulsant treatments should be considered.[5] Prochlorperazine and desferrioxamine may also interact with chlorpromazine to produce transient metabolic encephalopathy.[5]
Other drugs that prolong the QT interval, such as quinidine, verapamil, amiodarone, sotalol and methadone, may also interact with chlorpromazine to produce additive QT interval prolongation.[5]
Discontinuation
editThe British National Formulary recommends a gradual withdrawal when discontinuing antipsychotics to avoid acute withdrawal syndrome or rapid relapse.[34] Symptoms of withdrawal commonly include nausea, vomiting, and loss of appetite.[35] Other symptoms may include restlessness, increased sweating, and trouble sleeping.[35] Less commonly, there may be a feeling of the world spinning, numbness, or muscle pains.[35] Symptoms generally resolve after a short period of time.[35]
There is tentative evidence that discontinuation of antipsychotics can result in psychosis.[36] It may also result in reoccurrence of the condition that is being treated.[37] Rarely, tardive dyskinesia can occur when the medication is stopped.[35]
Pharmacology
editChlorpromazine is classified as a low-potency typical antipsychotic. Low-potency antipsychotics have more anticholinergic side effects, such as dry mouth, sedation, and constipation, and lower rates of extrapyramidal side effects, while high-potency antipsychotics (such as haloperidol) have the reverse profile.[16]
Pharmacodynamics
edit| Site | Ki | Species | Ref |
|---|---|---|---|
| 5-HT1A | 3115 | Human | [38] |
| 5-HT1B | 1,489 | Human | [39] |
| 5-HT1D | 452 | Human | [39] |
| 5-HT1E | 344 | Human | [39] |
| 5-HT2A | 2.75 | Human | [40] |
| 5-HT2C | 25 | Human | [41] |
| 5-HT3 | 776 | Human | [42] |
| 5-HT5A | 118 | Human | [39] |
| 5-HT6 | 19.5 | Human | [42] |
| 5-HT7 | 21 | Human | [39] |
| α1A | 0.28 | Human | [39] |
| α1B | 0.81 | Human | [39] |
| α2A | 184 | Human | [39] |
| α2B | 28 | Human | [39] |
| α2C | 46 | Human | [39] |
| β1 | >10,000 | Human | [39] |
| β2 | >10,000 | Human | [39] |
| M1 | 47 | Human | [39] |
| M2 | 433 | Human | [39] |
| M3 | 47 | Human | [39] |
| M4 | 151 | Human | [39] |
| D1 | 114.8 | Human | [42] |
| D2 | 7.244 | Human | [42] |
| D3 | 6.9 | Human | [43] |
| D4 | 32.36 | Human | [42] |
| H1 | 4.25 | Human | [43] |
| H2 | 174 | Human | [39] |
| H3 | 1,000 | Human | [43] |
| H4 | 5,048 | Human | [39] |
| NET | 2,443 | Human | [39] |
| DAT | >10,000 | Human | [39] |
Chlorpromazine is a very effective antagonist of D2 dopamine receptors and similar receptors, such as D3 and D5. Unlike most other drugs of this genre, it also has a high affinity for D1 receptors. Blocking these receptors causes diminished neurotransmitter binding in the forebrain, resulting in many different effects. Dopamine, unable to bind with a receptor, causes a feedback loop that causes dopaminergic neurons to release more dopamine. Therefore, upon first taking the drug, patients will experience an increase in dopaminergic neural activity. Eventually, dopamine production of the neurons will drop substantially and dopamine will be removed from the synaptic cleft. At this point, neural activity decreases greatly; the continual blockade of receptors only compounds this effect.[16]
Chlorpromazine acts as an antagonist (blocking agent) on different postsynaptic and presynaptic receptors:
- Dopamine receptors (subtypes D1, D2, D3 and D4), which account for its different antipsychotic properties on productive and unproductive symptoms, in the mesolimbic dopamine system accounts for the antipsychotic effect whereas the blockade in the nigrostriatal system produces the extrapyramidal effects
- Serotonin receptors (5-HT2, 5-HT6 and 5-HT7), with anxiolytic, antidepressant and antiaggressive properties as well as an attenuation of extrapyramidal side effects, but also leading to weight gain and ejaculation difficulties.
- Histamine receptors (H1 receptors, accounting for sedation, antiemetic effect, vertigo, and weight gain)
- α1- and α2-adrenergic receptors (accounting for sympatholytic properties, lowering of blood pressure, reflex tachycardia, vertigo, sedation, hypersalivation and incontinence as well as sexual dysfunction, but may also attenuate pseudoparkinsonism – controversial. Also associated with weight gain as a result of blockage of the adrenergic alpha 1 receptor as well as with intraoperative floppy iris syndrome due to its effect on the iris dilator muscle.[44]
- M1 and M2 muscarinic acetylcholine receptors (causing anticholinergic symptoms such as dry mouth, blurred vision, constipation, difficulty or inability to urinate, sinus tachycardia, electrocardiographic changes and loss of memory, but the anticholinergic action may attenuate extrapyramidal side effects).[medical citation needed]
The presumed effectiveness of the antipsychotic drugs relied on their ability to block dopamine receptors. This assumption arose from the dopamine hypothesis that maintains that both schizophrenia and bipolar disorder are a result of excessive dopamine activity. Furthermore, psychomotor stimulants like cocaine that increase dopamine levels can cause psychotic symptoms if taken in excess.[45]
Chlorpromazine and other typical antipsychotics are primarily blockers of D2 receptors. In fact an almost perfect correlation exists between the therapeutic dose of a typical antipsychotic and the drug's affinity for the D2 receptor. Therefore, a larger dose is required if the drug's affinity for the D2 receptor is relatively weak. A correlation exists between average clinical potency and affinity of the antipsychotics for dopamine receptors.[46] Chlorpromazine tends to have greater effect at serotonin receptors than at D2 receptors, which is notably the opposite effect of the other typical antipsychotics. Therefore, chlorpromazine with respect to its effects on dopamine and serotonin receptors is more similar to the atypical antipsychotics than to the typical antipsychotics.[46]
Chlorpromazine and other antipsychotics with sedative properties such as promazine and thioridazine are among the most potent agents at α-adrenergic receptors. Furthermore, they are also among the most potent antipsychotics at histamine H1 receptors. This finding is in agreement with the pharmaceutical development of chlorpromazine and other antipsychotics as anti-histamine agents. Furthermore, the brain has a higher density of histamine H1 receptors than any body organ examined which may account for why chlorpromazine and other phenothiazine antipsychotics are as potent at these sites as the most potent classical antihistamines.[47]
In addition to influencing the neurotransmitters dopamine, serotonin, epinephrine, norepinephrine, and acetylcholine it has been reported that antipsychotic drugs could achieve glutamatergic effects. This mechanism involves direct effects on antipsychotic drugs on glutamate receptors. By using the technique of functional neurochemical assay chlorpromazine and phenothiazine derivatives have been shown to have inhibitory effects on NMDA receptors that appeared to be mediated by action at the Zn site. It was found that there is an increase of NMDA activity at low concentrations and suppression at high concentrations of the drug. No significant difference in glycine activity from the effects of chlorpromazine were reported. Further work will be necessary to determine if the influence in NMDA receptors by antipsychotic drugs contributes to their effectiveness.[48]
Chlorpromazine does also act as a FIASMA (functional inhibitor of acid sphingomyelinase).[49]
Peripheral effects
editChlorpromazine is an antagonist to H1 receptors (provoking antiallergic effects), H2 receptors (reduction of forming of gastric juice), M1 and M2 receptors (dry mouth, reduction in forming of gastric juice) and some 5-HT receptors (different anti-allergic/gastrointestinal actions).[medical citation needed]
Because it acts on so many receptors, chlorpromazine is often referred to as a "dirty drug".[50]
Pharmacokinetics
edit| Bioavailability | tmax | CSS | Protein bound | Vd | t1/2 | Details of metabolism | Excretion | Notes |
|---|---|---|---|---|---|---|---|---|
| 10–80% | 1–4 hours (Oral); 6–24 hours (IM) | 100–300 ng/mL | 90–99% | 10–35 L/kg (mean: 22 L/kg) | 30±7 hours | CYP2D6, CYP1A2—mediated into over 10 major metabolites.[16] The major routes of metabolism include hydroxylation, N-oxidation, sulfoxidation, demethylation, deamination and conjugation. There is little evidence supporting the development of metabolic tolerance or an increase in the metabolism of chlorpromazine due to microsomal liver enzymes following multiple doses of the drug.[52] | Urine (43–65% after 24 hours) | Its high degree of lipophilicity (fat solubility) allows it to be detected in the urine for up to 18 months.[5][53] Less than 1% of the unchanged drug is excreted via the kidneys in the urine, in which 20–70% is excreted as conjugated or unconjugated metabolites, whereas 5–6% is excreted in feces.[53] |
History
editIn 1933, the French pharmaceutical company Laboratoires Rhône-Poulenc began to search for new antihistamines. In 1947, it synthesized promethazine, a phenothiazine derivative, which was found to have more pronounced sedative and antihistaminic effects than earlier drugs.[55]: 77 A year later, the French surgeon Pierre Huguenard used promethazine together with pethidine as part of a cocktail to induce relaxation and indifference in surgical patients. Another surgeon, Henri Laborit, believed the compound stabilized the central nervous system by causing "artificial hibernation" and described this state as "sedation without narcosis". He suggested to Rhône-Poulenc that they develop a compound with better stabilizing properties.[56] In December 1950, the chemist Paul Charpentier produced a series of compounds that included RP4560 or chlorpromazine.[7]
Chlorpromazine was distributed for testing to physicians between April and August 1951. Laborit trialled the medicine on at the Val-de-Grâce military hospital in Paris, using it as an anaesthetic booster in intravenous doses of 50 to 100 mg on surgery patients and confirming it as the best drug to date in calming and reducing shock, with patients reporting improved well being afterwards. He also noted its hypothermic effect and suggested it may induce artificial hibernation. Laborit thought this would allow the body to better tolerate major surgery by reducing shock, a novel idea at the time. Known colloquially as "Laborit's drug", chlorpromazine was released onto the market in 1953 by Rhône-Poulenc and given the trade name Largactil, derived from large "broad" and acti* "activity".[7]
Following on, Laborit considered whether chlorpromazine may have a role in managing patients with severe burns, Raynaud's phenomenon, or psychiatric disorders. At the Villejuif Mental Hospital in November 1951, he and Montassut administered an intravenous dose to psychiatrist Cornelia Quarti, who was acting as a volunteer. Quarti noted the indifference, but fainted upon getting up to go to the toilet, and so further testing was discontinued. (Orthostatic hypotension is a known side effect of chlorpromazine). Despite this, Laborit continued to push for testing in psychiatric patients during early 1952. Psychiatrists were reluctant initially, but on 19 January 1952, it was administered (alongside pethidine, pentothal and ECT) to Jacques Lh., a 24-year-old manic patient, who responded dramatically; he was discharged after three weeks, having received 855 mg of the drug in total.[7]
Pierre Deniker had heard about Laborit's work from his brother-in-law, who was a surgeon, and ordered chlorpromazine for a clinical trial at the Sainte-Anne Hospital Center in Paris where he was chief of the men's service.[7] Together with the hospital director Jean Delay, they published their first clinical trial in 1952, in which they treated thirty-eight psychotic patients with daily injections of chlorpromazine without the use of other sedating agents.[57] The response was dramatic; treatment with chlorpromazine went beyond simple sedation, with patients showing improvements in thinking and emotional behaviour.[58] They also found that doses higher than those used by Laborit were required, giving patients 75–100 mg daily.[7]
Deniker then visited America, where the publication of their work alerted the American psychiatric community that the new treatment might represent a real breakthrough. Heinz Lehmann of the Verdun Protestant Hospital in Montreal trialled it in seventy patients and also noted its striking effects, with patients' symptoms resolving after many years of unrelenting psychosis.[59] By 1954, chlorpromazine was being used in the United States to treat schizophrenia, mania, psychomotor excitement, and other psychotic disorders.[16][60][61] Rhône-Poulenc licensed chlorpromazine to Smith Kline & French (today's GlaxoSmithKline) in 1953. In 1955 it was approved in the United States for the treatment of emesis (vomiting). The effect of this drug in emptying psychiatric hospitals has been compared to that of penicillin on infectious diseases.[57]
But the popularity of the drug fell from the late 1960s as newer drugs came on the scene. From chlorpromazine a number of other similar antipsychotics were developed, leading to the discovery of antidepressants.[62]
Chlorpromazine largely replaced electroconvulsive therapy, hydrotherapy,[63] psychosurgery, and insulin shock therapy.[58] By 1964, about fifty million people worldwide had taken it.[64] Chlorpromazine, in widespread use for fifty years, remains a "benchmark" drug in the treatment of schizophrenia, an effective drug although not a perfect one.[29]
Society and culture
editIn literature
editThorazine was often depicted in Tom Wolfe's The Electric Kool-Aid Acid Test to abort bad trips on LSD. Thorazine is also mentioned in Fear and Loathing in Las Vegas, where it was reported to negate the effects of LSD.
Names
editBrand names include Thorazine, Largactil, Hibernal, and Megaphen (sold by Bayer in West-Germany since July 1953).[65]
Research
editChlorpromazine has tentative benefit[clarification needed] in animals infected with Naegleria fowleri[66] and shows antifungal and antibacterial activity in vitro.[67][clarification needed]
Veterinary use
editThe veterinary use of chlorpromazine has generally been superseded by use of acepromazine.[68]
Chlorpromazine may be used as an antiemetic in dogs and cats, or, less often, as sedative before anesthesia.[69] In horses, it often causes ataxia and lethargy and is therefore seldom used.[68][69]
It is commonly used to decrease nausea in animals that are too young for other common antiemetics.[citation needed] It is sometimes used as a preanesthetic and muscle relaxant in cattle, swine, sheep, and goats.[citation needed]
The use of chlorpromazine in food-producing animals is not permitted in the European Union, as a maximum residue limit could not be determined following assessment by the European Medicines Agency.[70]
References
edit- ^ "Chlorpromazine Pregnancy and Breastfeeding Warnings". Drugs.com. 5 February 2020. Retrieved 21 August 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ "List of nationally authorised medicinal products - Active substance: chlorpromazine: Procedure no.: PSUSA/00000715/202005" (PDF). Ema.europa.eu. Retrieved 3 March 2022.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z "Australian Product Information – Largactil (chlorpromazine hydrochloride)" (PDF). Therapeutic Goods Administration (TGA). Sanofi Aventis Pty Ltd. 28 August 2012. Archived from the original on 30 March 2017. Retrieved 8 December 2013.
- ^ a b c d e f g h i j k l m "Chlorpromazine Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 8 December 2015. Retrieved 1 December 2015.
- ^ a b c d e f López-Muñoz F, Alamo C, Cuenca E, Shen WW, Clervoy P, Rubio G (2005). "History of the discovery and clinical introduction of chlorpromazine". Annals of Clinical Psychiatry. 17 (3): 113–135. doi:10.1080/10401230591002002. PMID 16433053.
- ^ Ban TA (August 2007). "Fifty years chlorpromazine: a historical perspective". Neuropsychiatric Disease and Treatment. 3 (4): 495–500. PMC 2655089. PMID 19300578.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ López-Muñoz F, Alamo C, Cuenca E, Shen WW, Clervoy P, Rubio G (2005). "History of the discovery and clinical introduction of chlorpromazine". Annals of Clinical Psychiatry. 17 (3): 113–135. doi:10.1080/10401230591002002. PMID 16433053.
- ^ Shorter E (2005). A historical dictionary of psychiatry. New York: Oxford University Press. p. 6. ISBN 9780198039235. Archived from the original on 14 February 2017.
- ^ Douglas-Hall P, Whicher EV, et al. (Cochrane Schizophrenia Group) (December 2015). "'As required' medication regimens for seriously mentally ill people in hospital". The Cochrane Database of Systematic Reviews. 2015 (12): CD003441. doi:10.1002/14651858.CD003441.pub3. PMID 26689942.
- ^ Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. (September 2013). "Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis". Lancet. 382 (9896): 951–962. doi:10.1016/S0140-6736(13)60733-3. PMID 23810019. S2CID 32085212.
- ^ Adams CE, Awad GA, Rathbone J, Thornley B, Soares-Weiser K, et al. (Cochrane Schizophrenia Group) (January 2014). "Chlorpromazine versus placebo for schizophrenia". The Cochrane Database of Systematic Reviews. 1 (1): CD000284. doi:10.1002/14651858.CD000284.pub3. PMC 10640712. PMID 24395698.
- ^ a b c d e f g h i Brunton L, Chabner B, Knollman B (2010). Goodman and Gilman's The Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill Professional. ISBN 978-0-07-162442-8.
- ^ American Society of Health-System Pharmacists (1 November 2008). "Chlorpromazine". PubMed Health. National Center for Biotechnology Information. Archived from the original on 6 July 2010.
- ^ Breitbart W, Marotta R, Platt MM, Weisman H, Derevenco M, Grau C, et al. (February 1996). "A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients". The American Journal of Psychiatry. 153 (2): 231–237. doi:10.1176/ajp.153.2.231. PMID 8561204.
- ^ a b "Chlorpromazine". Martindale: The Complete Drug Reference. London: Pharmaceutical Press. 30 January 2013. Retrieved 8 December 2013.
- ^ Logan P, Loga P, Lewis D (April 2007). "Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Chlorpromazine in migraine". Emergency Medicine Journal. 24 (4): 297–300. doi:10.1136/emj.2007.047860. PMC 2658244. PMID 17384391.
- ^ Richter PA, Burk MP (July–August 1992). "The potentiation of narcotic analgesics with phenothiazines". The Journal of Foot Surgery. 31 (4): 378–380. PMID 1357024.
- ^ "Propaphenin, Medicine and Disease information". EPG Online. 14 July 2001. Archived from the original on 2 December 2013. Retrieved 26 November 2013.
- ^ a b Halman A, Kong G, Sarris J, Perkins D (January 2024). "Drug-drug interactions involving classic psychedelics: A systematic review". J Psychopharmacol. 38 (1): 3–18. doi:10.1177/02698811231211219. PMC 10851641. PMID 37982394.
- ^ Yates G, Melon E (January 2024). "Trip-killers: a concerning practice associated with psychedelic drug use". Emerg Med J. 41 (2): 112–113. doi:10.1136/emermed-2023-213377. PMID 38123961.
- ^ Suran M (February 2024). "Study Finds Hundreds of Reddit Posts on "Trip-Killers" for Psychedelic Drugs". JAMA. 331 (8): 632–634. doi:10.1001/jama.2023.28257. PMID 38294772.
- ^
- Henry M, Alibert S, Rogier C, Barbe J, Pradines B (1 April 2008). "Inhibition of efflux of quinolines as new therapeutic strategy in malaria". Current Topics in Medicinal Chemistry. 8 (7). Bentham: 563–578. doi:10.2174/156802608783955593. PMID 18473883. S2CID 13127221.
- Miki A, Tanabe K, Nakayama T, Kiryon C, Ohsawa K (March 1992). "Plasmodium chabaudi: association of reversal of chloroquine resistance with increased accumulation of chloroquine in resistant parasites". Experimental Parasitology. 74 (2). AP: 134–142. doi:10.1016/0014-4894(92)90040-h. PMID 1740175. S2CID 37364349.
- ^
- • Mangoni AA, Tuccinardi T, Collina S, Vanden Eynde JJ, Muñoz-Torrero D, Karaman R, et al. (June 2018). "Breakthroughs in Medicinal Chemistry: New Targets and Mechanisms, New Drugs, New Hopes-3". Molecules. 23 (7). MDPI AG: 1596. doi:10.3390/molecules23071596. PMC 6099979. PMID 29966350. S2CID 49644934.
- • Weeks JC, Roberts WM, Leasure C, Suzuki BM, Robinson KJ, Currey H, et al. (January 2018). "Sertraline, Paroxetine, and Chlorpromazine Are Rapidly Acting Anthelmintic Drugs Capable of Clinical Repurposing". Scientific Reports. 8 (1). Springer Science and Business Media LLC: 975. Bibcode:2018NatSR...8..975W. doi:10.1038/s41598-017-18457-w. PMC 5772060. PMID 29343694. S2CID 205636792.
- ^ a b Verlinden H, Vleugels R, Marchal E, Badisco L, Pflüger HJ, Blenau W, et al. (August 2010). "The role of octopamine in locusts and other arthropods". Journal of Insect Physiology. 56 (8). Elsevier: 854–867. Bibcode:2010JInsP..56..854V. doi:10.1016/j.jinsphys.2010.05.018. PMID 20621695.
- ^ a b c Adams CE, Awad GA, Rathbone J, Thornley B, Soares-Weiser K (January 2014). "Chlorpromazine versus placebo for schizophrenia". The Cochrane Database of Systematic Reviews. 1 (1): CD000284. doi:10.1002/14651858.CD000284.pub3. PMC 10640712. PMID 24395698. Archived from the original on 1 October 2015.
- ^ Pisani F, Oteri G, Costa C, Di Raimondo G, Di Perri R (2002). "Effects of psychotropic drugs on seizure threshold". Drug Safety. 25 (2): 91–110. doi:10.2165/00002018-200225020-00004. PMID 11888352. S2CID 25290793.
- ^ Leucht C, Kitzmantel M, Chua L, Kane J, Leucht S (January 2008). Leucht C (ed.). "Haloperidol versus chlorpromazine for schizophrenia". The Cochrane Database of Systematic Reviews (1): CD004278. doi:10.1002/14651858.CD004278.pub2. PMID 18254045.
- ^ Leucht S, Wahlbeck K, Hamann J, Kissling W (May 2003). "New generation antipsychotics versus low-potency conventional antipsychotics: a systematic review and meta-analysis". Lancet. 361 (9369): 1581–1589. doi:10.1016/S0140-6736(03)13306-5. PMID 12747876. S2CID 40851775.
- ^ Thomas D, Wu K, Kathöfer S, Katus HA, Schoels W, Kiehn J, et al. (June 2003). "The antipsychotic drug chlorpromazine inhibits HERG potassium channels". British Journal of Pharmacology. 139 (3): 567–574. doi:10.1038/sj.bjp.0705283. PMC 1573882. PMID 12788816.
- ^ Joint Formulary Committee B, ed. (March 2009). "4.2.1". British National Formulary (57 ed.). United Kingdom: Royal Pharmaceutical Society of Great Britain. p. 192. ISBN 978-0-85369-845-6.
Withdrawal of antipsychotic drugs after long-term therapy should always be gradual and closely monitored to avoid the risk of acute withdrawal syndromes or rapid relapse.
- ^ a b c d e Haddad PM, Dursun S, Deakin B (2004). Adverse Syndromes and Psychiatric Drugs: A Clinical Guide. OUP Oxford. pp. 207–16. ISBN 9780198527480.
- ^ Moncrieff J (July 2006). "Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse". Acta Psychiatrica Scandinavica. 114 (1): 3–13. doi:10.1111/j.1600-0447.2006.00787.x. PMID 16774655. S2CID 6267180.
- ^ Sacchetti E, Vita A, Siracusano A, Fleischhacker W (2013). Adherence to Antipsychotics in Schizophrenia. Springer Science & Business Media. p. 85. ISBN 9788847026797.
- ^ Maheux J, Ethier I, Rouillard C, Lévesque D (April 2005). "Induction patterns of transcription factors of the nur family (nurr1, nur77, and nor-1) by typical and atypical antipsychotics in the mouse brain: implication for their mechanism of action". The Journal of Pharmacology and Experimental Therapeutics. 313 (1): 460–473. doi:10.1124/jpet.104.080184. hdl:20.500.11794/17025. PMID 15615863. S2CID 1436507.
- ^ a b c d e f g h i j k l m n o p q r s t "Chlorpromazine". PDSP Database.
- ^ Gillman PK (October 2005). "Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity". British Journal of Anaesthesia. 95 (4): 434–441. doi:10.1093/bja/aei210. PMID 16051647.
- ^ Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, et al. (March 2003). "H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs". Neuropsychopharmacology. 28 (3): 519–526. doi:10.1038/sj.npp.1300027. PMID 12629531.
- ^ a b c d e Silvestre JS, Prous J (June 2005). "Research on adverse drug events. I. Muscarinic M3 receptor binding affinity could predict the risk of antipsychotics to induce type 2 diabetes". Methods and Findings in Experimental and Clinical Pharmacology. 27 (5): 289–304. doi:10.1358/mf.2005.27.5.908643. PMID 16082416.
- ^ a b c von Coburg Y, Kottke T, Weizel L, Ligneau X, Stark H (January 2009). "Potential utility of histamine H3 receptor antagonist pharmacophore in antipsychotics". Bioorganic & Medicinal Chemistry Letters. 19 (2): 538–542. doi:10.1016/j.bmcl.2008.09.012. PMID 19091563.
- ^ Tsai LM (2021). Lens and cataract. San Francisco: American Academy of Ophthalmology. p. 162. ISBN 978-1681044491.
- ^ Girault JA, Greengard P (May 2004). "The neurobiology of dopamine signaling". Archives of Neurology. 61 (5): 641–644. doi:10.1001/archneur.61.5.641. PMID 15148138.
- ^ a b McKim WA (2007). Drugs and behavior: an introduction to behavioral pharmacology (6th ed.). Upper Saddle River, New Jersey: Prentice Hall. p. 416. ISBN 978-0-13-219788-5.
- ^ Peroutka SJ, Synder SH (December 1980). "Relationship of neuroleptic drug effects at brain dopamine, serotonin, alpha-adrenergic, and histamine receptors to clinical potency". The American Journal of Psychiatry. 137 (12): 1518–1522. doi:10.1176/ajp.137.12.1518. PMID 6108081.
- ^ Lidsky TI, Yablonsky-Alter E, Zuck LG, Banerjee SP (August 1997). "Antipsychotic drug effects on glutamatergic activity". Brain Research. 764 (1–2): 46–52. doi:10.1016/S0006-8993(97)00423-X. PMID 9295192. S2CID 37454572.
- ^ Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, et al. (2011). Riezman H (ed.). "Identification of novel functional inhibitors of acid sphingomyelinase". PLOS ONE. 6 (8): e23852. Bibcode:2011PLoSO...623852K. doi:10.1371/journal.pone.0023852. PMC 3166082. PMID 21909365.
- ^ Falkai P, Vogeley K (April 2000). "[The chances of new atypical substances]". Fortschritte der Neurologie-Psychiatrie. 68 (Suppl 1). biopsychiatry.com: S32–S37. PMID 10907611. Archived from the original on 24 July 2010. Retrieved 6 July 2010.
- ^ "Chlorpromazine Hydrochloride 100mg/5ml Oral Syrup – Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Rosemont Pharmaceuticals Limited. 6 August 2013. Archived from the original on 11 December 2013. Retrieved 8 December 2013.
- ^ Dahl SG, Strandjord RE (April 1977). "Pharmacokinetics of chlorpromazine after single and chronic dosage". Clinical Pharmacology and Therapeutics. 21 (4): 437–448. doi:10.1002/cpt1977214437. PMID 849674. S2CID 6645825.
- ^ a b Yeung PK, Hubbard JW, Korchinski ED, Midha KK (1993). "Pharmacokinetics of chlorpromazine and key metabolites". European Journal of Clinical Pharmacology. 45 (6): 563–569. doi:10.1007/BF00315316. PMID 8157044. S2CID 6410850.
- ^ "Thorazine advertisement". Smith Kline & French. c. 1963.
When the patient lashes out against 'them' – Thorazine (brand of chlorpromazine) quickly puts an end to his violent outburst. 'Thorazine' is especially effective when the psychotic episode is triggered by delusions or hallucinations. At the outset of treatment, Thorazine's combination of antipsychotic and sedative effects provides both emotional and physical calming. Assaultive or destructive behavior is rapidly controlled. As therapy continues, the initial sedative effect gradually disappears. But the antipsychotic effect continues, helping to dispel or modify delusions, hallucinations and confusion, while keeping the patient calm and approachable. Smith Kline and French Laboratories
- ^ Healy D (2004). "Explorations in a new world". The creation of psychopharmacology. Harvard University Press. p. 77. ISBN 978-0-674-01599-9. Archived from the original on 8 September 2017. Retrieved 26 November 2013.
- ^ Healy D (2004). "Explorations in a new world". The creation of psychopharmacology. Harvard University Press. p. 80. ISBN 978-0-674-01599-9.
- ^ a b Turner T (January 2007). "Chlorpromazine: unlocking psychosis". BMJ. 334 (Suppl 1): s7. doi:10.1136/bmj.39034.609074.94. PMID 17204765. S2CID 33739419.
- ^ a b Healy D (2004). The Creation of Psychopharmacology. Harvard University Press. pp. 37–73. ISBN 978-0-674-01599-9. Archived from the original on 8 September 2017. Retrieved 26 November 2013.
- ^ Dronsfield A. "Chlorpromazine - unlocks the saylum". RSC Education. Retrieved 13 January 2022.
- ^ Long JW (1992). The Essential guide to prescription drugs. New York: HarperPerennial. pp. 321–25. ISBN 978-0-06-271534-0.
- ^ Reines BP (1990). "The Relationship Between Laboratory and Clinical Studies in Psychopharmacologic Discovery". Perspectives on Medical Research. 2. Medical Research Modernization Society. Archived from the original on 7 September 2015. Retrieved 26 November 2013.
- ^ Healy D (2004). "Introduction". The Creation of Psychopharmacology. Harvard University Press. p. 2. ISBN 9780674015999. Archived from the original on 8 September 2017. Retrieved 26 November 2013.
- ^ Healy D (2000). "Psychopharmacology and the Government of the Self" (PDF). davidhealy.org. Archived from the original (PDF) on 6 October 2014. Retrieved 20 July 2015.
- ^ "Drug for treating schizophrenia identified". PBS.org. WGBH-TV. Archived from the original on 18 September 2009. Retrieved 7 July 2010.
- ^ Bangen, Hans (1992). Geschichte der medikamentösen Therapie der Schizophrenie. Verlag für Wissenschaft und Bildung. p. 98. ISBN 3-927-408-82-4.
- ^ Kim JH, Jung SY, Lee YJ, Song KJ, Kwon D, Kim K, et al. (November 2008). "Effect of therapeutic chemical agents in vitro and on experimental meningoencephalitis due to Naegleria fowleri". Antimicrobial Agents and Chemotherapy. 52 (11): 4010–4016. doi:10.1128/AAC.00197-08. PMC 2573150. PMID 18765686.
- ^ Afeltra J, Verweij PE (July 2003). "Antifungal activity of nonantifungal drugs". European Journal of Clinical Microbiology & Infectious Diseases. 22 (7). Springer Nature: 397–407. doi:10.1007/s10096-003-0947-x. PMID 12884072. S2CID 10489462.
- ^ a b Plumb DC (2015). Plumb's Veterinary Drug Handbook (8th ed.). John Wiley & Sons. ISBN 978-1118911921.
- ^ a b Posner LA, Burns P (2009). "Chapter 13: Sedative agents: tranquilizers, alpha-2 agonists, and related agents". In Riviere JE, Papich MG, Adams RH (eds.). Veterinary pharmacology and therapeutics (9 ed.). Ames, Iowa: Wiley-Blackwell. pp. 337–80. ISBN 9780813820613.
- ^ "Chlorpromazine: summary report" (PDF). European Medicines Agency. Committee for Veterinary Medicinal Products. June 1996. Archived (PDF) from the original on 18 January 2017. Retrieved 17 January 2017.