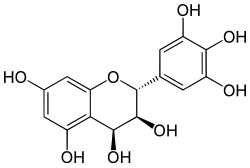
Leucodelphinidin is a colorless chemical compound related to leucoanthocyanidins. It can be found in Acacia auriculiformis,[1] in the bark of Karada (Cleistanthus collinus) and in the kino (gum) from Eucalyptus pilularis.[2]
| |
| Names | |
|---|---|
| IUPAC name
(2R,3S,4S)-Flavan-3,3′,4,4′,5,5′,7-heptol
| |
| Systematic IUPAC name
(2R,3S,4S)-2-(3,4,5-Trihydroxyphenyl)-2H-1-benzopyran-3,4,5,7-tetrol | |
| Other names
leukoefdin
Leucoefdin leucodelfinidin Leucoephdine Leukoephdin | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H14O8 | |
| Molar mass | 322.26 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Other species containing leucodelphinidin include Aesculus hippocastanum (Horse chestnut, in rind/bark/cortex), Arachis hypogaea (Earth nut in seeds), Arbutus unedo (Arbutus, in the leaf), Caesalpinia pulcherrima (Barbados pride), Ceratonia siliqua (Carob, in the fruit), Hamamelis virginiana (American witch hazel, in the leaf), Hippophae rhamnoides (Hippophae berry, in the leaf), Humulus lupulus (bine flower / blossom, in the leaf), Musa acuminata × balbisiana (Banana, in the fruit), Nelumbo nucifera (lotus, in the leaf), Phyllanthus emblica (Emblic, Indian gooseberry, in the rind/bark/cortex), Quercus alba (White oak, in the rind/bark/cortex), Quercus robur (Common oak, in the rind/bark/cortex), Rumex hymenosepalus (Arizona dock, in the root), Schinus molle (California peppertree, in the leaf) and Vicia faba (bell-bean, in the seed).[3]
A leucodelphinidin derivative isolated from Ficus bengalensis shows hypoglycemic effects.[4]
Metabolism
editDihydroflavonol 4-reductase (DFR) uses dihydromyricetin (ampelopsin) NADPH and 2 H+ to produce leucodelphinidin and NADP.[5][6]
References
edit- ^ Drewes, S. E.; Roux, D. G. (1966). "A new flavan-3,4-diol from Acacia auriculiformis by paper ionophoresis". Biochemical Journal. 98 (2): 493–500. doi:10.1042/bj0980493. PMC 1264869. PMID 5941342.
- ^ Ganguly, A.K.; Seshadri, T.R.; Subramanian, P. (1958). "A study of leucoanthocyanidins of plants—I". Tetrahedron. 3 (3): 225–229. doi:10.1016/0040-4020(58)80017-4.
- ^ Leucodelphinidin on liberherbarum.com
- ^ Geetha, B. S.; Mathew, B. C.; Augusti, K. T. (1994). "Hypoglycemic effects of leucodelphinidin derivative isolated from Ficus bengalensis (Linn)". Indian Journal of Physiology and Pharmacology. 38 (3): 220–222. PMID 7814088.
- ^ leucodelphinidin biosynthesis pathway proposed on biocyc.org
- ^ Les cibles d’amélioration pour la qualité des raisins: L’exemple des flavonoïdes, Nancy Terrier (French)[permanent dead link]