Betacoronavirus cameli [1] ( also known as Middle East respiratory syndrome–related coronavirus abbreviated as MERS-CoV),[2] or EMC/2012 (HCoV-EMC/2012), is the virus that causes Middle East respiratory syndrome (MERS).[3][4] It is a species of coronavirus which infects humans, bats, and camels.[5] The infecting virus is an enveloped, positive-sense, single-stranded RNA virus which enters its host cell by binding to the DPP4 receptor.[6] The species is a member of the genus Betacoronavirus and subgenus Merbecovirus.[7][5]
| Betacoronavirus cameli | |
|---|---|
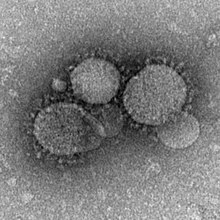
| |
| MERS-CoV particles as seen by negative stain electron microscopy. Virions contain characteristic club-like projections emanating from the viral membrane. | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Pisuviricota |
| Class: | Pisoniviricetes |
| Order: | Nidovirales |
| Family: | Coronaviridae |
| Genus: | Betacoronavirus |
| Subgenus: | Merbecovirus |
| Species: | Betacoronavirus cameli
|
| Synonyms | |
| |
Initially called simply novel coronavirus or nCoV, with the provisional names 2012 novel coronavirus (2012-nCoV) and human coronavirus 2012 (HCoV-12 or hCoV-12), it was first reported in June 2012 after genome sequencing of a virus isolated from sputum samples from a person who fell ill in a 2012 outbreak of a new flu-like respiratory illness. By July 2015, MERS-CoV cases had been reported in over 21 countries, in Europe, North America and Asia as well as the Middle East. MERS-CoV is one of several viruses identified by the World Health Organization (WHO) as a likely cause of a future epidemic. They list it for urgent research and development.[8][9]
Virology
editThe virus MERS-CoV is a member of the beta group of coronavirus, Betacoronavirus, lineage C. MERS-CoV genomes are phylogenetically classified into two clades, clade A and B. The earliest cases were of clade A clusters, while the majority of more recent cases are of the genetically distinct clade B.[10]
MERS-CoV is one of seven known coronaviruses to infect humans, including HCoV-229E, HCoV-NL63, HCoV-OC43, HCoV-HKU1, the original SARS-CoV (or SARS-CoV-1), and SARS-CoV-2.[11] It has frequently been referred to as a SARS-like virus.[12] By November, 2019, 2,494 cases of MERS had been reported with 858 deaths, implying a case fatality rate of greater than 30%.[13]
Early cases and spillover event
editThe first confirmed case was reported in Jeddah, Saudi Arabia in April 2012.[11] Egyptian virologist Ali Mohamed Zaki isolated and identified a previously unknown coronavirus from the man's lungs.[14][15][16] Zaki then posted his findings on 24 September 2012 on ProMED-mail.[15][17] The isolated cells showed cytopathic effects (CPE), in the form of rounding and syncytia formation.[17]
A second case was found in September 2012, when a 49-year-old man living in Qatar presented with similar flu symptoms. A sequence of the virus was nearly identical to that of the first case.[11] In November 2012, similar cases appeared in Qatar and Saudi Arabia. Additional cases were noted, with deaths associated, and rapid research and monitoring of the novel coronavirus began. It is not known whether the infections are the result of a single zoonotic event with subsequent human-to-human transmission, or if the multiple geographic sites of infection represent multiple zoonotic events from an unknown common source.[citation needed]
A study by Ziad Memish of Riyadh University and colleagues suggests that the virus arose some time between July 2007 and June 2012, with perhaps as many as seven separate zoonotic transmissions.[citation needed] Among animal reservoirs, CoV has a large genetic diversity yet the samples from patients suggested a similar genome, and therefore common source, though the data were limited. It was determined through molecular clock analysis that viruses from the EMC/2012 and England/Qatar/2012 date to early 2011, suggesting that these cases were descended from a single zoonotic event. It appeared the MERS-CoV had been circulating in the human population for more than a year without detection, and suggested independent transmission from an unknown source.[18][19]
Tropism
editIn humans, the virus has a strong tropism for nonciliated bronchial epithelial cells, and it has been shown to effectively evade the innate immune responses and antagonize interferon (IFN) production in these cells. This tropism is unique in that most respiratory viruses target ciliated cells.[20][21]
Due to the clinical similarity between MERS-CoV and SARS-CoV, it was proposed that they may use the same cellular receptor; the exopeptidase, angiotensin converting enzyme 2 (ACE2).[22] However, it was later discovered that neutralization of ACE2 by recombinant antibodies does not prevent MERS-CoV infection.[23] Further research identified dipeptidyl peptidase 4 (DPP4; also known as CD26) as a functional cellular receptor for MERS-CoV.[21] Unlike other known coronavirus receptors, the enzymatic activity of DPP4 is not required for infection. As would be expected, the amino acid sequence of DPP4 is highly conserved across species and is expressed in the human bronchial epithelium and kidneys.[21][24] Bat DPP4 genes appear to have been subject to a high degree of adaptive evolution as a response to coronavirus infections, so the lineage leading to MERS-CoV may have circulated in bat populations for a long period of time before being transmitted to people.[25]
Transmission
editOn 13 February 2013, the World Health Organization stated that "the risk of sustained person-to-person transmission appears to be very low."[26] The cells MERS-CoV infects in the lungs only account for 20% of respiratory epithelial cells, so a large number of virions are likely needed to be inhaled to cause infection.[24]
Anthony Fauci of the National Institutes of Health in Bethesda, Maryland, stated that MERS-CoV "does not spread in a sustained person to person way at all," while noting the possibility that the virus could mutate into a strain that does transmit from person to person.[27] However, the infection of healthcare workers has led to concerns of human to human transmission.[28]
The Centers for Disease Control and Prevention (CDC) list MERS as transmissible from human to human.[29] They state that "MERS-CoV has been shown to spread between people who are in close contact. Transmission from infected patients to healthcare personnel has also been observed. Clusters of cases in several countries are being investigated."[29][30]
However, on the 28th of May, the CDC revealed that the Illinois man who was originally thought to have been the first incidence of person-to-person spread (from the Indiana man at a business meeting), had in fact tested negative for MERS-CoV. After completing additional and more definitive tests using a neutralising antibody assay, experts at the CDC concluded that the Indiana patient did not spread the virus to the Illinois patient. Tests concluded that the Illinois man had not been previously infected. It is possible for MERS to be symptomless, and early research has shown that up to 20% of cases show no signs of active infection but have MERS-CoV antibodies in their blood.[31]
Evolution
editThe virus appears to have originated in bats.[32] The virus itself has been isolated from a bat.[33] This virus is closely related to the Tylonycteris bat coronavirus HKU4 and Pipistrellus bat coronavirus HKU5.[34] Serological evidence shows that these viruses have infected camels for at least 20 years. The most recent common ancestor of several human strains has been dated to March 2012 (95% confidence interval December 2011 to June 2012).[35]
It is thought that the viruses have been present in bats for some time and had spread to camels by the mid-1990s. The viruses appear to have spread from camels to humans in the early 2010s. The original bat host species and the time of initial infection in this species has yet to be determined.[citation needed] Examination of the sequences of 238 isolates suggested that this virus has evolved into three clades differing in codon usage, host, and geographic distribution.[36]
Natural reservoir
editIt is believed that the virus originated in bats, one candidate being the Egyptian tomb bat.[37] Work by epidemiologist Ian Lipkin of Columbia University in New York showed that the virus isolated from a bat looked to be a match to the virus found in humans.[33][38] 2c betacoronaviruses were detected in Nycteris bats in Ghana and Pipistrellus bats in Europe that are phylogenetically related to the MERS-CoV virus.[39] However the major natural reservoir where humans get the virus infection remained unknown until on 9 August 2013, a report in the journal The Lancet Infectious Diseases showed that 50 out of 50 (100%) blood serum from Omani camels and 15 of 105 (14%) from Spanish camels had protein-specific antibodies against the MERS-CoV spike protein. Blood serum from European sheep, goats, cattle, and other camelids had no such antibodies.[40]
Soon after on 5 September 2013 a seroepidemiological study published in the journal of Eurosurveillance by R.A Perera et al.[41] where they investigated 1343 human and 625 animal sera indicated, the abundant presence of MERS-CoV specific antibody in 108 out of 110 Egyptian dromedary camels but not in other animals such as goats, cows or sheep in this region.[41] These are the first and significant scientific reports that indicated the role of "dromedary camels" as a reservoir of MERS-CoV.[citation needed]
Research has linked camels, showing that the coronavirus infection in dromedary camel calves and adults is a 99.9% match to the genomes of human clade B MERS-CoV.[42] At least one person who has fallen sick with MERS was known to have come into contact with camels or recently drank camel milk.[43] Countries like Saudi Arabia and the United Arab Emirates produce and consume large amounts of camel meat. The possibility exists that African or Australian bats harbor the virus and transmit it to camels. Imported camels from these regions might have carried the virus to the Middle East.[44]
In 2013 MERS-CoV was identified in three members of a dromedary camel herd held in a Qatar barn, which was linked to two confirmed human cases who have since recovered. The presence of MERS-CoV in the camels was confirmed by the National Institute of Public Health and Environment (RIVM) of the Ministry of Health and the Erasmus Medical Center (WHO Collaborating Center), the Netherlands. None of the camels showed any sign of disease when the samples were collected. The Qatar Supreme Council of Health advised in November 2013 that people with underlying health conditions, such as heart disease, diabetes, kidney disease, respiratory disease, the immunosuppressed, and the elderly, avoid any close animal contacts when visiting farms and markets, and to practice good hygiene, such as washing hands.[45]
A further study on dromedary camels from Saudi Arabia published in December 2013 revealed the presence of MERS-CoV in 90% of the evaluated dromedary camels (310), suggesting that dromedary camels not only could be the main reservoir of MERS-CoV, but also the animal source of MERS.[46]
According to the 27 March 2014 MERS-CoV summary update, recent studies support that camels serve as the primary source of the MERS-CoV infecting humans, while bats may be the ultimate reservoir of the virus. Evidence includes the frequency with which the virus has been found in camels to which human cases have been exposed, seriological data which shows widespread transmission in camels, and the similarity of the camel CoV to the human CoV.[47]
On 6 June 2014, the Arab News newspaper highlighted the latest research findings in the New England Journal of Medicine in which a 44-year-old Saudi man who kept a herd of nine camels died of MERS in November 2013. His friends said they witnessed him applying a topical medicine to the nose of one of his ill camels—four of them reportedly sick with nasal discharge—seven days before he himself became stricken with MERS. Researchers sequenced the virus found in one of the sick camels and the virus that killed the man, and found that their genomes were identical. In that same article, the Arab News reported that as of 6 June 2014, there have been 689 cases of MERS reported within the Kingdom of Saudi Arabia with 283 deaths.[48]
Taxonomy
editMERS-CoV is more closely related to the bat coronaviruses HKU4 and HKU5 (lineage 2C) than it is to SARS-CoV (lineage 2B) (2, 9), sharing more than 90% sequence identity with their closest relationships, bat coronaviruses HKU4 and HKU5 and therefore considered to belong to the same species by the International Committee on Taxonomy of Viruses (ICTV).[citation needed]
- Mnemonic:
- Taxon identifier:
- Scientific name: Betacoronavirus cameli [1]
- Common name: Middle East respiratory syndrome coronavirus ( MERS-CoV) [2]
- Synonym: Severe acute respiratory syndrome coronavirus
- Other names:
- novel coronavirus (nCoV)
- London1 novel CoV/2012[49]
- Human Coronavirus Erasmus Medical Center/2012 (HCoV-EMC/2012)
- Rank:
- Lineage:
- › Viruses
- › ssRNA viruses
- › Group: IV; positive-sense, single-stranded RNA viruses
- › Order: Nidovirales
- › Family: Coronaviridae
- › Subfamily: Coronavirinae
- › Genus: Betacoronavirus[50]
- › Species: Betacoronavirus 1 (commonly called Human coronavirus OC43), Human coronavirus HKU1, Murine coronavirus, Pipistrellus bat coronavirus HKU5, Rousettus bat coronavirus HKU9, Severe acute respiratory syndrome-related coronavirus, Tylonycteris bat coronavirus HKU4, MERS-CoV, Severe acute respiratory syndrome coronavirus 2
- › Genus: Betacoronavirus[50]
- › Subfamily: Coronavirinae
- › Family: Coronaviridae
- › Order: Nidovirales
- › Group: IV; positive-sense, single-stranded RNA viruses
- › ssRNA viruses
Strains:
- Isolate:
- Isolate:
- NCBI
Research and patent
editSaudi officials had not given permission for Dr. Zaki, the first isolator of the human strain, to send a sample of the virus to Fouchier and were angered when Fouchier claimed the patent on the full genetic sequence of MERS-CoV.[54]
The editor of The Economist observed, "Concern over security must not slow urgent work. Studying a deadly virus is risky. Not studying it is riskier."[54] Dr. Zaki was fired from his job at the hospital as a result of bypassing the Saudi Ministry of Health in his announcement and sharing his sample and findings.[55][56][57][58]
At their annual meeting of the World Health Assembly in May 2013, WHO chief Margaret Chan declared that intellectual property, or patents on strains of new virus, should not impede nations from protecting their citizens by limiting scientific investigations. Deputy Health Minister Ziad Memish raised concerns that scientists who held the patent for MERS-CoV would not allow other scientists to use patented material and were therefore delaying the development of diagnostic tests.[59] Erasmus MC responded that the patent application did not restrict public health research into MERS and MERS-CoV,[60] and that the virus and diagnostic tests were shipped—free of charge—to all that requested such reagents.
Mapping
editThere are a number of mapping efforts focused on tracking MERS coronavirus. On 2 May 2014, the Corona Map[61] was launched to track the MERS coronavirus in realtime on the world map. The data is officially reported by WHO or the Ministry of Health of the respective country.[62] HealthMap also tracks case reports with inclusion of news and social media as data sources as part of HealthMap MERS.[63] South Korea was infected in mid-2015, with 38 deaths among 186 cases of infection.[citation needed]
See also
editReferences
edit- ^ a b "Taxon Details | ICTV". International Committee on Taxonomy of Viruses (ICTV). Retrieved 25 July 2024.
- ^ a b de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, Fouchier RA, Galiano M, Gorbalenya AE, Memish ZA, Perlman S, Poon LL, Snijder EJ, Stephens GM, Woo PC, Zaki AM, Zambon M, Ziebuhr J (July 2013). "Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group". Journal of Virology. 87 (14): 7790–2. doi:10.1128/JVI.01244-13. PMC 3700179. PMID 23678167.
- ^ "Middle East respiratory syndrome coronavirus (MERS-CoV)". www.who.int. Archived from the original on 18 April 2018. Retrieved 15 April 2020.
- ^ Zumla A, Hui DS, Perlman S (September 2015). "Middle East respiratory syndrome". Lancet. 386 (9997): 995–1007. doi:10.1016/S0140-6736(15)60454-8. PMC 4721578. PMID 26049252.
- ^ a b Wong AC, Li X, Lau SK, Woo PC (20 February 2019). "Global Epidemiology of Bat Coronaviruses". Viruses. 11 (2): 174. doi:10.3390/v11020174. PMC 6409556. PMID 30791586.
See Figure 3.
- ^ Fehr AR, Perlman S (2015). "Coronaviruses: An Overview of Their Replication and Pathogenesis". In Maier HJ, Bickerton E, Britton P (eds.). Coronaviruses. Methods in Molecular Biology. Vol. 1282. Springer. pp. 1–23. doi:10.1007/978-1-4939-2438-7_1. ISBN 978-1-4939-2438-7. PMC 4369385. PMID 25720466.
See Table 1.
- ^ "Virus Taxonomy: 2018 Release". International Committee on Taxonomy of Viruses (ICTV). October 2018. Archived from the original on 20 March 2020. Retrieved 13 January 2019.
- ^ Kieny MP. "After Ebola, a Blueprint Emerges to Jump-Start R&D". Scientific American Blog Network. Archived from the original on 20 December 2016. Retrieved 13 December 2016.
- ^ "LIST OF PATHOGENS". World Health Organization. Archived from the original on 20 December 2016. Retrieved 13 December 2016.
- ^ Chu DK, Poon LL, Gomaa MM, Shehata MM, Perera RA, Abu Zeid D, El Rifay AS, Siu LY, Guan Y, Webby RJ, Ali MA, Peiris M, Kayali G (June 2014). "MERS Coronaviruses in Dromedary Camels, Egypt". Emerging Infectious Diseases. 20 (6): 1049–1053. doi:10.3201/eid2006.140299. PMC 4036765. PMID 24856660.
- ^ a b c "ECDC Rapid Risk Assessment - Severe respiratory disease associated with a novel coronavirus" (PDF). 19 February 2013. Archived from the original (PDF) on 31 May 2013. Retrieved 22 April 2014.
- ^ Saey TH (2013). "Story one: Scientists race to understand deadly new virus: SARS-like infection causes severe illness, but may not spread quickly among people". Science News. 183 (6): 5–6. doi:10.1002/scin.5591830603. PMC 7169524. PMID 32327842.
- ^ "Middle East Respiratory Syndrome Coronavirus MERS-CoV". WHO. November 2019. Archived from the original on 18 October 2019. Retrieved 20 July 2020.
- ^ Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA (November 2012). "Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia". The New England Journal of Medicine. 367 (19): 1814–20. doi:10.1056/NEJMoa1211721. PMID 23075143. S2CID 7671909.
- ^ a b Falco M (24 September 2012). "New SARS-like virus poses medical mystery". CNN. Archived from the original on 27 September 2012. Retrieved 27 September 2012.
- ^ Dziadosz A (13 May 2013). "The doctor who discovered a new SARS-like virus says it will probably trigger an epidemic at some point, but not necessarily in its current form". Reuters. Archived from the original on 11 March 2016. Retrieved 25 May 2013.
- ^ a b "See Also". ProMED-mail. 20 September 2012. Archived from the original on 27 May 2013. Retrieved 31 May 2013.
- ^ Cotten M, Lam TT, Watson SJ, Palser AL, Petrova V, Grant P, Pybus OG, Rambaut A, Guan Y, Pillay D, Kellam P, Nastouli E (19 May 2013). "Full-Genome Deep Sequencing and Phylogenetic Analysis of Novel Human Betacoronavirus - Vol. 19 No. 5 - May 2013 - CDC". Emerging Infectious Diseases. 19 (5): 736–42B. doi:10.3201/eid1905.130057. PMC 3647518. PMID 23693015.
- ^ Lau SK, Lee P, Tsang AK, Yip CC, Tse H, Lee RA, So LY, Lau YL, Chan KH, Woo PC, Yuen KY (November 2011). "Molecular epidemiology of human coronavirus OC43 reveals evolution of different genotypes over time and recent emergence of a novel genotype due to natural recombination". Journal of Virology. 85 (21): 11325–37. doi:10.1128/JVI.05512-11. PMC 3194943. PMID 21849456.
- ^ Kindler E, Jónsdóttir HR, Muth D, Hamming OJ, Hartmann R, Rodriguez R, Geffers R, Fouchier RA, Drosten C, Müller MA, Dijkman R, Thiel V (February 2013). "Efficient replication of the novel human betacoronavirus EMC on primary human epithelium highlights its zoonotic potential". mBio. 4 (1): e00611–12. doi:10.1128/mBio.00611-12. PMC 3573664. PMID 23422412.
- ^ a b c Raj VS, Mou H, Smits SL, Dekkers DH, Müller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL (March 2013). "Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC". Nature. 495 (7440): 251–4. Bibcode:2013Natur.495..251R. doi:10.1038/nature12005. PMC 7095326. PMID 23486063.
- ^ Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB (December 2005). "ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia". Journal of Virology. 79 (23): 14614–21. doi:10.1128/JVI.79.23.14614-14621.2005. PMC 1287568. PMID 16282461.
- ^ Müller MA, Raj VS, Muth D, Meyer B, Kallies S, Smits SL, Wollny R, Bestebroer TM, Specht S, Suliman T, Zimmermann K, Binger T, Eckerle I, Tschapka M, Zaki AM, Osterhaus AD, Fouchier RA, Haagmans BL, Drosten C (December 2012). "Human coronavirus EMC does not require the SARS-coronavirus receptor and maintains broad replicative capability in mammalian cell lines". mBio. 3 (6). doi:10.1128/mBio.00515-12. PMC 3520110. PMID 23232719.
- ^ a b Butler D (13 March 2013). "Receptor for new coronavirus found". Nature. 495 (7440): 149–150. Bibcode:2013Natur.495..149B. doi:10.1038/495149a. PMID 23486032.
- ^ Cui J, Eden JS, Holmes EC, Wang LF (October 2013). "Adaptive evolution of bat dipeptidyl peptidase 4 (dpp4): implications for the origin and emergence of Middle East respiratory syndrome coronavirus". Virology Journal. 10: 304. doi:10.1186/1743-422X-10-304. PMC 3852826. PMID 24107353.
- ^ WHO: Novel coronavirus infection – update (13 February 2013) (accessed 13 February 2013)
- ^ "Fauci: New Virus Not Yet a 'threat to the world' (video)". Washington Times. 31 August 2012. Archived from the original on 15 April 2023. Retrieved 31 May 2013.
- ^ Knickmeyer E, Al Omran A (20 April 2014). "Concerns Spread as New Saudi MERS Cases Spike". Wall Street Journal. Archived from the original on 21 April 2014. Retrieved 22 April 2014.
- ^ a b "MERS-CoV – Frequently Asked Questions and Answers - Coronavirus". U.S. Centers for Disease Control and Prevention. 14 September 2017. Archived from the original on 15 March 2023. Retrieved 10 September 2017.
- ^ Grady D (19 June 2013). "Investigation Follows Trail of a Virus in Hospitals". The New York Times. Archived from the original on 19 January 2022. Retrieved 27 February 2017.
- ^ Aleccia J (28 May 2014). "CDC Backtracks: Illinois Man Didn't Have MERS After All". Archived from the original on 1 June 2014. Retrieved 2 June 2014.
- ^ Corman VM, Ithete NL, Richards LR, Schoeman MC, Preiser W, Drosten C, Drexler JF (October 2014). "Rooting the phylogenetic tree of middle East respiratory syndrome coronavirus by characterization of a conspecific virus from an African bat". Journal of Virology. 88 (19): 11297–303. doi:10.1128/JVI.01498-14. PMC 4178802. PMID 25031349.
- ^ a b Memish ZA, Mishra N, Olival KJ, Fagbo SF, Kapoor V, Epstein JH, Alhakeem R, Durosinloun A, Al Asmari M, Islam A, Kapoor A, Briese T, Daszak P, Al Rabeeah AA, Lipkin WI (November 2013). "Middle East respiratory syndrome coronavirus in bats, Saudi Arabia". Emerging Infectious Diseases. 19 (11): 1819–23. doi:10.3201/eid1911.131172. PMC 3837665. PMID 24206838.
- ^ Wang Q, Qi J, Yuan Y, Xuan Y, Han P, Wan Y, Ji W, Li Y, Wu Y, Wang J, Iwamoto A, Woo PC, Yuen KY, Yan J, Lu G, Gao GF (September 2014). "Bat origins of MERS-CoV supported by bat coronavirus HKU4 usage of human receptor CD26". Cell Host & Microbe. 16 (3): 328–37. doi:10.1016/j.chom.2014.08.009. PMC 7104937. PMID 25211075.
- ^ Cotten M, Watson SJ, Zumla AI, Makhdoom HQ, Palser AL, Ong SH, Al Rabeeah AA, Alhakeem RF, Assiri A, Al-Tawfiq JA, Albarrak A, Barry M, Shibl A, Alrabiah FA, Hajjar S, Balkhy HH, Flemban H, Rambaut A, Kellam P, Memish ZA (February 2014). "Spread, circulation, and evolution of the Middle East respiratory syndrome coronavirus". mBio. 5 (1): e01062–13. doi:10.1128/mBio.01062-13. PMC 3944817. PMID 24549846.
- ^ Alnazawi M, Altaher A, Kandeel M (2017). "Comparative Genomic Analysis MERS CoV Isolated from Humans and Camels with Special Reference to Virus Encoded Helicase". Biological & Pharmaceutical Bulletin. 40 (8): 1289–1298. doi:10.1248/bpb.b17-00241. PMID 28769010.
- ^ Mohd HA, Al-Tawfiq JA, Memish ZA (December 2016). "Middle East Respiratory Syndrome Coronavirus (MERS-CoV) origin and animal reservoir". Virology Journal. 13 (1): 87. doi:10.1186/s12985-016-0544-0. PMC 4891877. PMID 27255185.
- ^ Mole B (23 August 2013). "Deadly coronavirus found in bats". Nature. doi:10.1038/nature.2013.13597. S2CID 87673446. Archived from the original on 8 March 2021. Retrieved 19 January 2014.
- ^ a b Annan A, Baldwin HJ, Corman VM, Klose SM, Owusu M, Nkrumah EE, Badu EK, Anti P, Agbenyega O, Meyer B, Oppong S, Sarkodie YA, Kalko EK, Lina PH, Godlevska EV, Reusken C, Seebens A, Gloza-Rausch F, Vallo P, Tschapka M, Drosten C, Drexler JF (March 2013). "Human betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe". Emerging Infectious Diseases. 19 (3): 456–9. doi:10.3201/eid1903.121503. PMC 3647674. PMID 23622767.
- ^ Reusken CB, Haagmans BL, Müller MA, Gutierrez C, Godeke GJ, Meyer B, Muth D, Raj VS, Smits-De Vries L, Corman VM, Drexler JF, Smits SL, El Tahir YE, De Sousa R, van Beek J, Nowotny N, van Maanen K, Hidalgo-Hermoso E, Bosch BJ, Rottier P, Osterhaus A, Gortázar-Schmidt C, Drosten C, Koopmans MP (October 2013). "Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study". The Lancet. Infectious Diseases. 13 (10): 859–66. doi:10.1016/S1473-3099(13)70164-6. hdl:10261/142869. PMC 7106530. PMID 23933067.
- ^ a b Perera R, Wang P, Gomaa M, El-Shesheny R, Kandeil A, Bagato O, Siu L, Shehata M, Kayed A, Moatasim Y, Li M, Poon L, Guan Y, Webby R, Ali M, Peiris J, Kayali G (2013). "Eurosurveillance - Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013". Eurosurveillance. 18 (36): 20574. doi:10.2807/1560-7917.ES2013.18.36.20574. PMID 24079378.
- ^ Hemida MG, Chu DK, Poon LL, Perera RA, Alhammadi MA, Ng HY, Siu LY, Guan Y, Alnaeem A, Peiris M (July 2014). "MERS coronavirus in dromedary camel herd, Saudi Arabia". Emerging Infectious Diseases. 20 (7): 1231–4. doi:10.3201/eid2007.140571. PMC 4073860. PMID 24964193.
- ^ Roos R (17 April 2014). "MERS outbreaks grow; Malaysian case had camel link". Archived from the original on 23 April 2014. Retrieved 22 April 2014.
- ^ "Camels May Transmit New Middle Eastern Virus". 8 August 2013. Archived from the original on 9 August 2013. Retrieved 8 August 2013.
- ^ "Three camels hit by MERS coronavirus in Qatar". Qatar Supreme Council of Health. Archived from the original on 4 December 2013. Retrieved 28 November 2013.
- ^ Hemida MG, Perera RA, Wang P, Alhammadi MA, Siu LY, Li M, Poon LL, Saif L, Alnaeem A, Peiris M (December 2013). "Middle East Respiratory Syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013". Euro Surveillance. 18 (50): 20659. doi:10.2807/1560-7917.es2013.18.50.20659. PMID 24342517.
- ^ "Middle East respiratory syndrome coronavirus (MERS-CoV)Summary and literature update – as of 27 March 2014" (PDF). 27 March 2014. Archived (PDF) from the original on 24 April 2014. Retrieved 24 April 2014.
- ^ Fakeih MR (6 June 2014). "80% drop in MERS infections". Arab News. XXXIX (183): 1.
- ^ Roos R (25 September 2013). UK agency picks name for new coronavirus isolate (Report). University of Minnesota, Minneapolis, MN: Center for Infectious Disease Research & Policy (CIDRAP). Archived from the original on 6 May 2013. Retrieved 24 May 2013.
- ^ a b Bermingham A, Chand MA, Brown CS, Aarons E, Tong C, Langrish C, Hoschler K, Brown K, Galiano M, Myers R, Pebody RG, Green HK, Boddington NL, Gopal R, Price N, Newsholme W, Drosten C, Fouchier RA, Zambon M (October 2012). "Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012" (PDF). Euro Surveillance. 17 (40): 20290. PMID 23078800. Archived (PDF) from the original on 12 October 2022. Retrieved 19 January 2014.
- ^ Doucleff M (28 September 2012). "Holy Bat Virus! Genome Hints At Origin Of SARS-Like Virus". NPR. Archived from the original on 29 September 2012. Retrieved 29 September 2012.
- ^ Abedine S (13 March 2013). "Death toll from new SARS-like virus climbs to 9". CNN. Archived from the original on 13 March 2013. Retrieved 13 March 2013.
- ^ "New Coronavirus Has Many Potential Hosts, Could Pass from Animals to Humans Repeatedly". ScienceDaily. Archived from the original on 15 December 2012. Retrieved 13 December 2012.
- ^ a b "Pandemic preparedness: Coming, ready or not". The Economist. 20 April 2013. Archived from the original on 16 May 2013. Retrieved 5 September 2017.
- ^ "Egyptian Virologist Who Discovered New SARS-Like Virus Fears Its Spread". Mpelembe. 13 May 2013. Archived from the original on 29 June 2013. Retrieved 25 May 2013.
- ^ Sample I, Smith M (15 March 2013). "Coronavirus victim's widow tells of grief as scientists scramble for treatment". The Guardian. Archived from the original on 30 September 2013. Retrieved 25 May 2013.
- ^ Sample I (15 March 2013). "Coronavirus: Is this the next pandemic?". The Guardian. Archived from the original on 30 September 2013. Retrieved 25 May 2013.
- ^ Yang J (21 October 2012). "How medical sleuths stopped a deadly new SARS-like virus in its tracks". Toronto Star. Archived from the original on 13 May 2013. Retrieved 25 May 2013.
- ^ "Erasmus MC: no restrictions for public health research into MERS coronavirus". Erasmus MC (Press release). Rotterdam. 24 May 2013. Archived from the original on 17 June 2015. Retrieved 28 June 2013.
- ^ "CoronaMap: Realtime tracking of MERS Corona virus on world map". coronamap.com. Archived from the original on 4 May 2014. Retrieved 16 February 2020.
- ^ "Corona Map" (Press release). 2 May 2014. Archived from the original on 4 May 2014. Retrieved 16 February 2020.
- ^ "MERS (global map of cases)". healthmap.org. Archived from the original on 31 October 2020. Retrieved 30 January 2020.
External links
edit- Emergence of the Middle East Respiratory Syndrome Coronavirus
- MERS-CoV Complete Genome
- Emerging viruses
- Molecular Illustration of MERS-Coronavirus
- Philippines still MERS-CoV free – DOH Archived 28 June 2014 at the Wayback Machine
- Deadly Middle East Coronavirus found in an Egyptian tomb bat