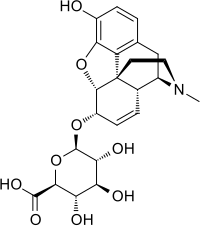
Morphine-6-glucuronide (M6G) is a major active metabolite of morphine. M6G is formed from morphine by the enzyme UGT2B7.[1] It has analgesic effects more potent than morphine.[2] M6G can accumulate to toxic levels in kidney failure.[3][4]
| |
| Names | |
|---|---|
| Other names
M6G
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.161.871 |
| MeSH | Morphine-6-glucuronide |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C23H27NO9 | |
| Molar mass | 461.46 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
History of discovery
editThis analgesic activity of M6G (in animals) was first noted by Yoshimura.[5]
Subsequent work at St Bartholomew's Hospital, London in the 1980s,[6] using a sensitive and specific high-performance liquid chromatography assay,[7] accurately defined for the first time the metabolism of morphine, and the abundance of this metabolite (along with morphine-3-glucuronide,[8] considered an inactive metabolite).
It was postulated that kidney impairment would result in accumulation of the kidney-excreted active agent M6G, leading to potentially fatal toxicity such as respiratory depression. The frequent use of morphine in critically ill patients, and the common occurrence of kidney failure in this group implied that M6G accumulation could be a common, but previously unanticipated problem. The first studies demonstrated massive levels of M6G in 3 patients with kidney failure, which resolved as kidney function returned.[3] Accumulation of M3G and M6G also decreased with return of kidney function after kidney transplantation.[4]
A key step in defining the importance of M6G in humans came in 1992 when the substance was artificially synthesised and administered to patients with pain, the majority of whom described pain relief.[9]
See also
editReferences
edit- ^ Coffman BL, Rios GR, King CD, Tephly TR (1 January 1997). "Human UGT2B7 catalyzes morphine glucuronidation". Drug Metab. Dispos. 25 (1): 1–4. PMID 9010622.
- ^ van Dorp EL, Romberg R, Sarton E, Bovill JG, Dahan A (2006). "Morphine-6-glucuronide: morphine's successor for postoperative pain relief?". Anesthesia and Analgesia. 102 (6): 1789–1797. doi:10.1213/01.ane.0000217197.96784.c3. PMID 16717327. S2CID 18890026.
- ^ a b Osborne, R J; Joel, SP; Slevin, ML (1986). "Morphine intoxication in renal failure: the role of morphine-6-glucuronide". Br Med J. 292 (6535): 1548–9. doi:10.1136/bmj.292.6535.1548. PMC 1340555. PMID 3087512.
- ^ a b Osborne, R; Joel, S; Grebenik, K; Trew, D; Slevin, M (1993). "The pharmacokinetics of morphine and morphine glucuronides in kidney failure". Clin Pharmacol Ther. 54 (2): 158–67. doi:10.1038/clpt.1993.127. PMID 8354025. S2CID 44954994.
- ^ Hidetoshi, Y; Oguri, K; Tsukamoto, H (1969). "Metabolism of drugs. LXII. Isolation and identification of morphine glucuronides in urine and bile of rabbits". Biochem Pharmacol. 18 (2): 279–86. doi:10.1016/0006-2952(69)90205-6. PMID 5778147.
- ^ Osborne, R; Joel, S; Trew, D; Slevin, M (1990). "Morphine and metabolite behavior after different routes of morphine administration: demonstration of the importance of the active metabolite morphine-6-glucuronide". Clin Pharmacol Ther. 47 (1): 12–9. doi:10.1038/clpt.1990.2. PMID 2295214. S2CID 37751253.
- ^ Joel, S; Osborne, RJ; Slevin, ML (1988). "An improved method for the simultaneous determination of morphine and its principal glucuronide metabolites". J Chromatogr. 430 (2): 394–9. doi:10.1016/S0378-4347(00)83176-X. PMID 3235512.
- ^ Renal tubular transport of morphine, morphine-6-glucuronide, and morphine-3-glucuronide in the isolated perfused rat kidney. JT Van Crugten, BC Sallustio, RL Nation and AA Somogyi. Department of Clinical and Experimental Pharmacology, University of Adelaide, Australia.
- ^ Osborne, R; Thompson, P; Joel, S; Trew, D; Patel, N; Slevin, M (1992). "The analgesic activity of morphine-6-glucuronide". Br J Clin Pharmacol. 34 (2): 130–8. doi:10.1111/j.1365-2125.1992.tb04121.x. PMC 1381529. PMID 1419474.