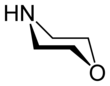
Morpholine is an organic chemical compound having the chemical formula O(CH2CH2)2NH. This heterocycle features both amine and ether functional groups. Because of the amine, morpholine is a base; its conjugate acid is called morpholinium. For example, treating morpholine with hydrochloric acid generates the salt morpholinium chloride. It is a colorless liquid with a weak, ammonia- or fish-like odor.[6] The naming of morpholine is attributed to Ludwig Knorr, who incorrectly believed it to be part of the structure of morphine.[7]
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Morpholine[2] | |||
| Other names
Diethylenimide oxide
1,4-Oxazinane Tetrahydro-1,4-oxazine Diethylene imidoxide Diethylene oximide Tetrahydro-p-oxazine | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 102549 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.469 | ||
| EC Number |
| ||
| 1803 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2054 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H9NO | |||
| Molar mass | 87.122 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Weak ammonia-like or fish-like[3] | ||
| Density | 1.007 g/cm3 | ||
| Melting point | −5 °C (23 °F; 268 K) | ||
| Boiling point | 129 °C (264 °F; 402 K) | ||
| miscible | |||
| Vapor pressure | 6 mmHg (20 °C)[3] | ||
| Acidity (pKa) | 8.36[4] (of conjugate acid) | ||
| -55.0·10−6 cm3/mol | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Flammable, Corrosive | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H226, H302, H312, H314, H332 | |||
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P363, P370+P378, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 31 °C (88 °F; 304 K) | ||
| 275 °C (527 °F; 548 K) | |||
| Explosive limits | 1.4%–11.2%[3] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1220 mg/kg (mammal, oral) 525 mg/kg (mouse, oral) 1050 mg/kg (rat, oral)[5] | ||
LC50 (median concentration)
|
365 ppm (mouse, 2 hr)[5] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 20 ppm (70 mg/m3) [skin][3] | ||
REL (Recommended)
|
TWA 20 ppm (70 mg/m3) ST 30 ppm (105 mg/m3) [skin][3] | ||
IDLH (Immediate danger)
|
1400 ppm[3] | ||
| Safety data sheet (SDS) | hazard.com | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Production
editMorpholine is often produced industrially by the dehydration of diethanolamine with concentrated sulfuric acid.[8] Alternatively, it can be made from bis(2-chloroethyl)ether in a reaction with ammonia, by which also ammonium chloride is formed.
Morpholine is also produced industrially from diethylene glycol and ammonia, under high temperature and pressure, in the presence of hydrogen and a suitable catalyst.[9]
Uses
editIndustrial applications
editMorpholine is a common additive, in parts per million concentrations, for pH adjustment in both fossil fuel and nuclear power plant steam systems. Morpholine is used because its volatility is about the same as water, so once it is added to the water, its concentration becomes distributed rather evenly in both the water and steam phases. Its pH-adjusting qualities then become distributed throughout the steam plant to provide corrosion protection. Morpholine is often used in conjunction with low concentrations of hydrazine or ammonia to provide a comprehensive all-volatile treatment chemistry for corrosion protection for the steam systems of such plants. Morpholine decomposes reasonably slowly in the absence of oxygen at the high temperatures and pressures in these steam systems.
Organic synthesis
editMorpholine undergoes most chemical reactions typical for other secondary amines, though the presence of the ether oxygen withdraws electron density from the nitrogen, rendering it less nucleophilic (and less basic) than structurally similar secondary amines such as piperidine. For this reason, it forms a stable chloramine.[10]
It is commonly used to generate enamines.[11]
Morpholine is widely used in organic synthesis. For example, it is a building block in the preparation of the antibiotic linezolid, the anticancer agent gefitinib (brand name Iressa) and the analgesic dextromoramide.
In research and in industry, the low cost and polarity of morpholine lead to its common use as a solvent for chemical reactions.
Agriculture
editAs a fruit coating
editIn nature, fruits make waxes to protect against insects and fungal contamination, but this can be lost as the fruit is cleaned. Hence a small amount of new wax, made from shellac, is applied to replace it. Morpholine is sometimes used as an emulsifier and solubility aid for this new coating.[12] The European Union has forbidden the use of morpholine in fruit coating.[13][14]
As a component in fungicides
editMorpholine derivatives used as agricultural fungicides in cereals are known as ergosterol biosynthesis inhibitors.
See also
editReferences
edit- ^ National Institute for Occupational Safety and Health (2000). "Morpholine". International Chemical Safety Cards. Retrieved 5 November 2005.
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 142. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0437". National Institute for Occupational Safety and Health (NIOSH).
- ^ Hall, H. K. (1957). "Correlation of the Base Strengths of Amines1". J. Am. Chem. Soc. 79 (20): 5441–5444. doi:10.1021/ja01577a030.
- ^ a b "Morpholine". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "CDC - NIOSH Pocket Guide to Chemical Hazards - Morpholine". www.cdc.gov. Retrieved 4 January 2022.
- ^ F. Silversmith, Ernest; Nickon, Alex (2013-10-22). Organic Chemistry : Modern Coined Terms and Their Origins. Elsevier Science. p. 313. ISBN 978-1483145235.
- ^ Weissermel, Klaus; Arpe, Hans-Jürgen; Lindley, Charlet R.; Hawkins, Stephen (2003). "Chapter 7. Oxidation Products of Ethylene". Industrial Organic Chemistry. Wiley-VCH. pp. 159–161. ISBN 3-527-30578-5.
- ^ U.S. Patent 3151112, "Process for the preparation of morpholines" van 29 september 1964 aan Jefferson Chemical Company.
- ^ Lindsay Smith, J. R.; McKeer, L. C.; Taylor, J. M. (1993). "4-Chlorination of Electron-Rich Benzenoid Compounds: 2,4-Dichloromethoxybenzene". Organic Syntheses; Collected Volumes, vol. 8, p. 167.
- ^ Noyori, R.; Yokoyama, K.; Hayakawa, Y. (1988). "Cyclopentenones from α,α′-Dibromoketones and Enamines: 2,5-Dimethyl-3-Phenyl-2-Cyclopenten-1-one". Organic Syntheses; Collected Volumes, vol. 6, p. 520.
- ^ McGuire, Raymond G.; Dimitroglou, Dimitrios A. (1999). "Evaluation of Shellac and Sucrose Ester Fruit Coating Formulations that Support Biological Control of Post-harvest Grapefruit Decay". Bio-control Science and Technology. 9 (1): 53–65. Bibcode:1999BioST...9...53M. doi:10.1080/09583159929901.
- ^ "Morpholine". Scientific Analysis Laboratories Ltd. Archived from the original on 2012-04-26.
- ^ "Morpholine Issues in the United Kingdom". Northwest Horticultural Council. September 28, 2010. Archived from the original on April 26, 2012.
External links
edit- Media related to Morpholines at Wikimedia Commons