Acetylleucine (N-acetyl-leucine) is a modified leucine amino acid used in the treatment of vertigo[2] and cerebellar ataxia.
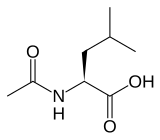 (S)-(−)-N-Acetyl-leucine
| |
| Names | |
|---|---|
| IUPAC name
2-Acetamido-4-methylpentanoic acid[1]
| |
| Other names
N-Acetylleucine; N-Acetyl-L-Leucine
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| 3DMet |
|
| 1724849 (S)-(−) | |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider | |
| EC Number |
|
| 985259 (S)-(−) | |
| KEGG |
|
| MeSH | acetylleucine |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C8H15NO3 | |
| Molar mass | 173.212 g·mol−1 |
| Appearance | White crystals |
| Melting point | −115 to −113 °C; −175 to −172 °F; 158 to 160 K |
| log P | −0.265 |
| Acidity (pKa) | 3.666 |
| Basicity (pKb) | 10.331 |
| Pharmacology | |
| N07CA04 (WHO) | |
| Related compounds | |
Related compounds
|
ENU |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Two forms exist: acetyl-DL-leucine (sold under the brand Tanganil, among others) and N-acetyl-L-leucine (levacetylleucine).[3]
Acetylleucine is also being developed as a possible treatment for several neurological disorders by IntraBio Inc.[4] Clinical trials with acetylleucine for the treatment of three orphan, fatal, neurodegenerative disorders are underway: Niemann-Pick disease type C,[5] GM2 gangliosidoses (Tay-Sachs and Sandhoff diseases),[6] and ataxia–telangiectasia.[7] In 2020, IntraBio announced the successful multinational clinical trial results of the Niemann-Pick type C clinical trial.[8] IntraBio is also investigating acetylleucine for the treatment of common inherited and acquired neurological diseases including Lewy body dementia,[9] amyotrophic lateral sclerosis, restless legs syndrome, multiple sclerosis, and migraine[10] Acetylleucine has received orphan drug designations from the U.S. Food & Drug Administration (FDA)[11][12][13][14] and the European Commission.[15][16][17][18]
References
edit- ^ "N-Acetyl-DL-leucine". PubChem Open Chemistry Database. Retrieved 26 March 2017.
- ^ "N07CA04 (acetylleucine)". WHO Collaborating Centre for Drug Statistics Methodology. Norwegian Institute of Public Health. 19 December 2016. Retrieved 26 March 2017.
- ^ Oertel WH, Janzen A, Henrich MT, Geibl FF, Sittig E, Meles SK, et al. (2 September 2024). "Acetyl-DL-leucine in two individuals with REM sleep behavior disorder improves symptoms, reverses loss of striatal dopamine-transporter binding and stabilizes pathological metabolic brain pattern—case reports". Nature Communications. 15 (1): 7619. doi:10.1038/s41467-024-51502-7. ISSN 2041-1723. PMC 11369233. PMID 39223119.
- ^ "IntraBio". Archived from the original on 1 August 2019. Retrieved 1 August 2019.
- ^ "N-Acetyl-L-Leucine for Niemann-Pick Disease, Type C (NPC) - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 1 August 2019.
- ^ "N-Acetyl-L-Leucine for GM2 Gangliosdisosis (Tay-Sachs and Sandhoff Disease) - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 1 August 2019.
- ^ "N-Acetyl-L-Leucine for Ataxia-Telangiectasia (A-T) - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 1 August 2019.
- ^ "IntraBio Reports Further Detail on Positive Data from IB1001 Multinational Clinical Trial for the Treatment of Niemann-Pick disease Type C". intrabio.com. 19 October 2020. Retrieved 1 August 2021.
- ^ Passmore P (15 April 2014). "A clinical trial to test amlodipine as a new treatment for vascular dementia". doi:10.1186/isrctn31208535.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Strupp M, Bayer O, Feil K, Straube A (1 February 2019). "Prophylactic treatment of migraine with and without aura with acetyl-dl-leucine: a case series". Journal of Neurology. 266 (2): 525–529. doi:10.1007/s00415-018-9155-6. ISSN 1432-1459. PMID 30547273. S2CID 56148131.
- ^ "Search Orphan Drug Designations and Approvals". www.accessdata.fda.gov. Retrieved 3 August 2019.
- ^ "Search Orphan Drug Designations and Approvals". www.accessdata.fda.gov. Retrieved 3 August 2019.
- ^ "Search Orphan Drug Designations and Approvals". www.accessdata.fda.gov. Retrieved 3 August 2019.
- ^ "Search Orphan Drug Designations and Approvals". www.accessdata.fda.gov. Retrieved 3 August 2019.
- ^ "Public Health - European Commission". Union Register of medicinal products. Retrieved 3 August 2019.
- ^ "Public Health - European Commission". Union Register of medicinal products. Retrieved 3 August 2019.
- ^ "Public Health - European Commission". Union Register of medicinal products. Retrieved 3 August 2019.
- ^ "Public Health - European Commission". Union Register of medicinal products. Retrieved 3 August 2019.