Neodymium(III) fluoride is an inorganic chemical compound of neodymium and fluorine with the formula NdF3. It is a purplish pink colored solid with a high melting point.
| |
| Names | |
|---|---|
| Other names
Neodymium trifluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.852 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| NdF3 | |
| Molar mass | 201.24 g/mol |
| Appearance | Vibrant pink/violet solid |
| Density | 6.5g/cm3 |
| Melting point | 1,374 °C (2,505 °F; 1,647 K) |
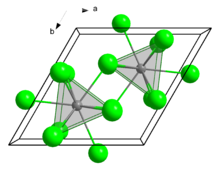
| Structure | |
| Tricapped trigonal prismatic (nine-coordinate) | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H312, H315, H319, H332, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
editLike other lanthanide fluorides it is highly insoluble in water which allows it to be synthesised from aqueous neodymium nitrate via a reaction with hydrofluoric acid, from which it precipitates as a hydrate:[1]
- Nd(NO3)3(aq) + 3 HF → NdF3•½H2O + 3 HNO3
It can also be obtained by the reaction of neodymium(III) oxide and hydrofluoric acid:[2]
- Nd2O3 + 6HF → 2NdF3 + 3H2O
Anhydrous material may be obtained by the simple drying of the hydrate, in contrast to the hydrates of other neodymium halides, which form mixed oxyhalides if heated.[1]
Uses
editNeodymium(III) fluoride is often used in the manufacture of fluoride glasses.[3]
When neodymium is extracted from ores, the fluoride is often an intermediate product and is then reduced to the solid metal chemically (e.g. by adding calcium, which produces calcium fluoride) or by fused-salt electrolysis.
Other compounds
editNeodymium(III) fluoride forms compounds with N2H4, such as NdF3•3N2H4•3H2O which is a white hexagonal crystal, soluble in water, slightly soluble in methanol and ethanol, with d20°C = 2.3547 g/cm3.[4]
References
edit- ^ a b Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the elements (2nd ed.). Butterworth-Heinemann. p. 1240. ISBN 0-7506-3365-4.
- ^ Georg Brauer (Hrsg.), unter Mitarbeit von Marianne Baudler u. a.: Handbuch der Präparativen Anorganischen Chemie. 3., umgearbeitete Auflage. Band I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6, S. 254.
- ^ Sigma-Aldrich Co., product no. {{{id}}}.
- ^ Izvestii︠a︡ vysshikh uchebnykh zavedeniĭ: Khimii︠a︡ i khimicheskai︠a︡ tekhnologii︠a︡, Tập 16,Số phát hành 1 (Ivanovskiĭ khimiko-tekhnologicheskiĭ in-t, 1973), pages 181–182. Retrieved 19 Jan 2021.