Periodic acid (/ˌpɜːraɪˈɒdɪk/ per-eye-OD-ik) is the highest oxoacid of iodine, in which the iodine exists in oxidation state +7. It can exist in two forms: orthoperiodic acid, with the chemical formula H5IO6, and metaperiodic acid, which has the formula HIO4.
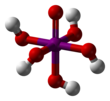
Orthoperiodic acid
| |||
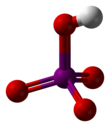
Metaperiodic acid
| |||
 Orthoperiodic acid
| |||
| Names | |||
|---|---|---|---|
Other names
| |||
| Identifiers | |||
| |||
3D model (JSmol)
|
| ||
| ChemSpider | |||
| ECHA InfoCard | 100.030.839 | ||
| EC Number |
| ||
PubChem CID
|
| ||
| UNII |
| ||
| UN number | UN3085 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| HIO4 (metaperiodic) H5IO6 (orthoperiodic) | |||
| Molar mass | 190.91 g/mol (HIO4) 227.941 g/mol (H5IO6) | ||
| Appearance | Colourless crystals | ||
| Melting point | 128.5 °C (263.3 °F; 401.6 K)[1] | ||
| Solubility | soluble in water, alcohols | ||
| Conjugate base | Periodate | ||
| Hazards[2] | |||
| GHS labelling: | |||
   
| |||
| Danger | |||
| H271, H314, H372, H400 | |||
| P210, P260, P273, P303+P361+P353, P305+P351+P338 | |||
| NFPA 704 (fire diamond) | |||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Other anions
|
| ||
Other cations
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Periodic acid was discovered by Heinrich Gustav Magnus and C. F. Ammermüller in 1833.[3]
Synthesis
editModern industrial scale production involves the oxidation of a solution of sodium iodate under alkaline conditions, either electrochemically on a PbO2 anode, or by treatment with chlorine:[4]
- IO−3 + 6 HO− - 2 e− → IO5−6 + 3 H2O (counter ions omitted for clarity) E° = -1.6 V[5]
- IO−3 + 6 HO− + Cl2 → IO5−6 + 2 Cl− + 3 H2O
A standard laboratory preparation involves treating a mixture of barium periodate with nitric acid. Upon concentrating the mixture, the barium nitrate, which is less soluble, is separated from periodic acid:[6]
- Ba3H4(IO6)2 + 6 HNO3 → 3 Ba(NO3)2 + 2H5IO6
Properties
editOrthoperiodic acid has a number of acid dissociation constants.[7][8] The pKa of metaperiodic acid has not been determined.
- H5IO6 ⇌ H4IO−6 + H+, pKa = 3.29
- H4IO−6 ⇌ H3IO2−6 + H+, pKa = 8.31
- H3IO2−6 ⇌ H2IO3−6 + H+, pKa = 11.60
There being two forms of periodic acid, it follows that two types of periodate salts are formed. For example, sodium metaperiodate, NaIO4, can be synthesised from HIO4 while sodium orthoperiodate, Na5IO6 can be synthesised from H5IO6.
Structure
editOrthoperiodic acid forms monoclinic crystals (space group P21/n) consisting of a slightly deformed IO6 octahedron interlinked via bridging hydrogens. Five I–O bond distances are in the range 1.87–1.91 Å and one I–O bond is 1.78 Å.[9][10] The structure of metaperiodic acid also includes IO6 octahedra, however these are connected via cis-edge-sharing with bridging oxygens to form one-dimensional infinite chains.[11]
Reactions
editOrthoperiodic acid can be dehydrated to give metaperiodic acid by heating to 100 °C under reduced pressure.
- H5IO6 ⇌ HIO4 + 2 H2O
Further heating to around 150 °C gives iodine pentoxide (I2O5) rather than the expected anhydride diiodine heptoxide (I2O7). Metaperiodic acid can also be prepared from various orthoperiodates by treatment with dilute nitric acid.[12]
Like all periodates periodic acid can be used to cleave various 1,2-difunctional compounds. Most notably periodic acid will cleave vicinal diols into two aldehyde or ketone fragments (Malaprade reaction).
This can be useful in determining the structure of carbohydrates as periodic acid can be used to open saccharide rings. This process is often used in labeling saccharides with fluorescent molecules or other tags such as biotin. Because the process requires vicinal diols, periodate oxidation is often used to selectively label the 3′-termini of RNA (ribose has vicinal diols) instead of DNA as deoxyribose does not have vicinal diols.
Periodic acid is also used as an oxidising agent of moderate strength, as exemplified in the Babler oxidation of secondary allyl alcohols which are oxidised to enones by stoichiometric amounts of orthoperiodic acid with catalyst PCC.[13]
Other oxyacids
editPeriodic acid is part of a series of oxyacids in which iodine can assume oxidation states of −1, +1, +3, +5, or +7. A number of neutral iodine oxides are also known.
| Iodine oxidation state | −1 | +1 | +3 | +5 | +7 |
|---|---|---|---|---|---|
| Name | Hydrogen iodide | Hypoiodous acid | Iodous acid | Iodic acid | Periodic acid |
| Formula | HI | HIO | HIO2 | HIO3 | HIO4 or H5IO6 |
See also
editCompounds with a similar structure:
- Perchloric acid, perbromic acid, the related perhalogenic acids
- Telluric acid and perxenic acid, the isoelectronic oxoacids of tellurium and xenon
Compounds with similar chemistry:
References
edit- ^ Aylett, founded by A.F. Holleman; continued by Egon Wiberg; translated by Mary Eagleson, William Brewer; revised by Bernhard J. (2001). Inorganic chemistry (1st English ed., [edited] by Nils Wiberg. ed.). San Diego, Calif. : Berlin: Academic Press, W. de Gruyter. p. 453. ISBN 0123526515.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ "Periodsaeure zur Synthese". Sigma Aldrich. 8 October 2021. Retrieved 21 November 2021.
- ^ Ammermüller, F.; Magnus, G. (1833). "Ueber eine neue Verbindung des Jods mit Sauerstoff, die Ueberjodsäure". Annalen der Physik und Chemie (in German). 104 (7): 514–525. Bibcode:1833AnP...104..514A. doi:10.1002/andp.18331040709.
- ^ Greenwood, N. N.; Earnshaw, A (1997). Chemistry of the elements (2nd ed.). Butterworth-Heinemann. p. 872. doi:10.1016/C2009-0-30414-6. ISBN 978-0-7506-3365-9.
- ^ Parsons, Roger (1959). Handbook of electrochemical constants. Butterworths Scientific Publications Ltd. p. 71.
- ^ M. Schmeisser (1963). "Periodic acid". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 2pages=322. NY,NY: Academic Press.
- ^ Aylett, founded by A.F. Holleman; continued by Egon Wiberg; translated by Mary Eagleson, William Brewer; revised by Bernhard J. (2001). Inorganic chemistry (1st English ed., [edited] by Nils Wiberg. ed.). San Diego, Calif. : Berlin: Academic Press, W. de Gruyter. p. 454. ISBN 0123526515.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Burgot, Jean-Louis (2012-03-30). Ionic equilibria in analytical chemistry. New York: Springer. p. 358. ISBN 978-1441983824.
- ^ Feikema, Y. D. (10 June 1966). "The crystal structures of two oxy-acids of iodine. I. A study of orthoperiodic acid, H5IO6, by neutron diffraction". Acta Crystallographica. 20 (6): 765–769. doi:10.1107/S0365110X66001828.
- ^ Fábry, J.; Podlahová, J.; Loub, J.; Langer, V. (1982). "Structure of the 1:1 adduct of orthoperiodic acid and urea". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry. 38 (3): 1048–1050. doi:10.1107/S0567740882004932.
- ^ Kraft, Thorsten; Jansen, Martin (1 September 1997). "Crystal Structure Determination of Metaperiodic Acid, HIO4, with Combined X-Ray and Neutron Diffraction". Angewandte Chemie International Edition in English. 36 (16): 1753–1754. doi:10.1002/anie.199717531.
- ^ Riley (1963). Brauer, Georg (ed.). Handbook of preparative inorganic chemistry. Volume 1. Translated by Scripta Technica, Inc. Translation editor Reed F. (2nd ed.). New York, N.Y.: Academic Press. pp. 323–324. ISBN 012126601X.
- ^ Killoran, Patrick M.; Rossington, Steven B.; Wilkinson, James A.; Hadfield, John A. (2016). "Expanding the scope of the Babler–Dauben oxidation: 1,3-oxidative transposition of secondary allylic alcohols". Tetrahedron Letters. 57 (35): 3954–3957. doi:10.1016/j.tetlet.2016.07.076.