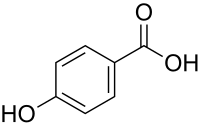
4-Hydroxybenzoic acid, also known as p-hydroxybenzoic acid (PHBA), is a monohydroxybenzoic acid, a phenolic derivative of benzoic acid. It is a white crystalline solid that is slightly soluble in water and chloroform but more soluble in polar organic solvents such as alcohols and acetone. 4-Hydroxybenzoic acid is primarily known as the basis for the preparation of its esters, known as parabens, which are used as preservatives in cosmetics and some ophthalmic solutions. It is isomeric with 2-hydroxybenzoic acid, known as salicylic acid, a precursor to aspirin, and with 3-hydroxybenzoic acid.
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Hydroxybenzoic acid | |
| Other names
p-Hydroxybenzoic acid
para-Hydroxybenzoic acid PHBA 4-hydroxybenzoate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.002.550 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H6O3 | |
| Molar mass | 138.122 g·mol−1 |
| Appearance | White crystalline solid |
| Odor | Odorless |
| Density | 1.46 g/cm3 |
| Melting point | 214.5 °C (418.1 °F; 487.6 K) |
| Boiling point | N/A, decomposes[1] |
| 0.5 g/100 mL | |
| Solubility |
|
| log P | 1.58 |
| Acidity (pKa) | 4.54 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| NFPA 704 (fire diamond) | |
| 250 °C (482 °F; 523 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2200 mg/kg (oral, mouse) |
| Safety data sheet (SDS) | HMDB |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Natural occurrences
editIt is found in plants of the genus Vitex such as V. agnus-castus or V. negundo, and in Hypericum perforatum (St John's wort). It is also found in Spongiochloris spongiosa, a freshwater green alga.
The compound is also found in Ganoderma lucidum, a medicinal mushroom with the longest record of use.
Cryptanaerobacter phenolicus is a bacterium species that produces benzoate from phenol via 4-hydroxybenzoate.[2]
Occurrences in food
edit4-Hydroxybenzoic acid can be found naturally in coconut.[3] It is one of the main catechins metabolites found in humans after consumption of green tea infusions.[4] It is also found in wine,[5] in vanilla, in Macrotyloma uniflorum (horse gram), carob[6] and in Phyllanthus acidus (Otaheite gooseberry).
Açaí oil, obtained from the fruit of the açaí palm (Euterpe oleracea), is rich in p-hydroxybenzoic acid (892±52 mg/kg).[7] It is also found in cloudy olive oil[citation needed] and in the edible mushroom Russula virescens (green-cracking russula).[citation needed]
Related compounds
editp-Hydroxybenzoic acid glucoside can be found in mycorrhizal and non-mycorrhizal roots of Norway spruces (Picea abies).[8]
Violdelphin is an anthocyanin, a type of plant pigments, found in blue flowers and incorporating two p-hydroxybenzoic acid residues, one rutinoside and two glucosides associated with a delphinidin.
Agnuside is the ester of aucubin and p-hydroxybenzoic acid.[9]
Biosynthesis
editChorismate lyase is an enzyme that transforms chorismate into 4-hydroxybenzoate and pyruvate. This enzyme catalyses the first step in ubiquinone biosynthesis in Escherichia coli and other Gram-negative bacteria.
Benzoate 4-monooxygenase is an enzyme that utilizes benzoate, NADPH, H+ and O2 to produce 4-hydroxybenzoate, NADP+ and H2O. This enzyme can be found in Aspergillus niger.
4-Hydroxybenzoate also arises from tyrosine.[10]
Metabolism
editAs an intermediate
editThe enzyme 4-methoxybenzoate monooxygenase (O-demethylating) transforms 4-methoxybenzoate, an electron acceptor AH2 and O2 into 4-hydroxybenzoate, formaldehyde, the reduction product A and H2O. This enzyme participates in 2,4-dichlorobenzoate degradation in Pseudomonas putida.
The enzyme 4-hydroxybenzaldehyde dehydrogenase uses 4-hydroxybenzaldehyde, NAD+ and H2O to produce 4-hydroxybenzoate, NADH and H+. This enzyme participates in toluene and xylene degradation in bacteria such as Pseudomonas mendocina. It is also found in carrots (Daucus carota).
The enzyme that 2,4'-dihydroxyacetophenone dioxygenase transforms 2,4'-dihydroxyacetophenone and O2 into 4-hydroxybenzoate and formate. This enzyme participates in bisphenol A degradation. It can be found in Alcaligenes species.
The enzyme 4-chlorobenzoate dehalogenase uses 4-chlorobenzoate and H2O to produce 4-hydroxybenzoate and chloride. It can be found in Pseudomonas species.
The enzyme 4-hydroxybenzoyl-CoA thioesterase utilizes 4-hydroxybenzoyl-CoA and H2O to produce 4-hydroxybenzoate and CoA. This enzyme participates in 2,4-dichlorobenzoate degradation. It can be found in Pseudomonas species.
The enzyme 4-hydroxybenzoate polyprenyltransferase uses a polyprenyl diphosphate and 4-hydroxybenzoate to produce diphosphate and 4-hydroxy-3-polyprenylbenzoate. This enzyme participates in ubiquinone biosynthesis.
The enzyme 4-hydroxybenzoate geranyltransferase utilizes geranyl diphosphate and 4-hydroxybenzoate to produce 3-geranyl-4-hydroxybenzoate and diphosphate. Biosynthetically, alkannin is produced in plants from the intermediates 4-hydroxybenzoic acid and geranyl pyrophosphate. This enzyme is involved in shikonin biosynthesis. It can be found in Lithospermum erythrorhizon.
The enzyme 3-hydroxybenzoate—CoA ligase uses ATP, 3-hydroxybenzoate and CoA to produce AMP, diphosphate and 3-hydroxybenzoyl-CoA. The enzyme works equally well with 4-hydroxybenzoate. It can be found in Thauera aromatica.
Biodegradation
editThe enzyme 4-hydroxybenzoate 1-hydroxylase transforms 4-hydroxybenzoate, NAD(P)H, 2 H+ and O2 into hydroquinone, NAD(P)+, H2O and CO2. This enzyme participates in 2,4-dichlorobenzoate degradation. It can be found in Candida parapsilosis.
The enzyme 4-hydroxybenzoate 3-monooxygenase transforms 4-hydroxybenzoate, NADPH, H+ and O2 into protocatechuate, NADP+ and H2O. This enzyme participates in benzoate degradation via hydroxylation and 2,4-dichlorobenzoate degradation. It can be found in Pseudomonas putida and Pseudomonas fluorescens.
The enzyme 4-hydroxybenzoate 3-monooxygenase (NAD(P)H) utilizes 4-hydroxybenzoate, NADH, NADPH, H+ and O2 to produce 3,4-dihydroxybenzoate (protocatechuic acid), NAD+, NADP+ and H2O. This enzyme participates in benzoate degradation via hydroxylation and 2,4-dichlorobenzoate degradation. It can be found in Corynebacterium cyclohexanicum and in Pseudomonas sp.
The enzyme 4-hydroxybenzoate decarboxylase uses 4-hydroxybenzoate to produce phenol and CO2. This enzyme participates in benzoate degradation via coenzyme A (CoA) ligation. It can be found in Klebsiella aerogenes (Aerobacter aerogenes).
The enzyme 4-hydroxybenzoate—CoA ligase transforms ATP, 4-hydroxybenzoate and CoA to produce AMP, diphosphate and 4-hydroxybenzoyl-CoA. This enzyme participates in benzoate degradation via CoA ligation. It can be found in Rhodopseudomonas palustris.
Coniochaeta hoffmannii is a plant pathogen that commonly inhabits fertile soil. It is known to metabolize aromatic compounds of low molecular weight, such as p-hydroxybenzoic acid.
Glycosylation
editThe enzyme 4-hydroxybenzoate 4-O-beta-D-glucosyltransferase transforms UDP-glucose and 4-hydroxybenzoate into UDP and 4-(beta-D-glucosyloxy)benzoate. It can be found in the pollen of Pinus densiflora.
Chemistry
editThe Hammett equation describes a linear free-energy relationship relating reaction rates and equilibrium constants for many reactions involving benzoic acid derivatives with meta- and para-substituents.
Chemical production
edit4-Hydroxybenzoic acid is produced commercially from potassium phenoxide and carbon dioxide in the Kolbe-Schmitt reaction.[11] It can also be produced in the laboratory by heating potassium salicylate with potassium carbonate to 240 °C, followed by treating with acid.[12]
Chemical reactions
edit4-Hydroxybenzoic acid has about one tenth the acidity of benzoic acid, having an acid dissociation constant Ka = 3.3×10−5 M at 19 °C.[citation needed] Its acid dissociation follows this equation:
- HOC6H4CO2H ⇌ HOC6H4CO−2 + H+
Chemical use
editVectran is a manufactured fiber, spun from a liquid crystal polymer. Chemically it is an aromatic polyester produced by the polycondensation of 4-hydroxybenzoic acid and 6-hydroxynaphthalene-2-carboxylic acid. The fiber has been shown to exhibit strong radiation shielding used by Bigelow Aerospace and produced by StemRad.[13]
4,4′-Dihydroxybenzophenone is generally prepared by the rearrangement of p-hydroxyphenylbenzoate. Alternatively, p-hydroxybenzoic acid can be converted to p-acetoxybenzoyl chloride. This acid chloride reacts with phenol to give, after deacetylation, 4,4′-dihydroxybenzophenone.
Examples of drugs made from PHBA include nifuroxazide, orthocaine, ormeloxifene and proxymetacaine.
Bioactivity and safety
edit4-Hydroxybenzoic acid is a popular antioxidant in part because of its low toxicity. The LD50 is 2200 mg/kg in mice (oral).[14]
4-Hydroxybenzoic acid has estrogenic activity both in vitro and in vivo,[15] and stimulates the growth of human breast cancer cell lines.[16][17] It is a common metabolite of paraben esters, such as methylparaben.[15][16][17] The compound is a relatively weak estrogen, but can produce uterotrophy with sufficient doses to an equivalent extent relative to estradiol, which is unusual for a weakly estrogenic compound and indicates that it may be a full agonist of the estrogen receptor with relatively low binding affinity for the receptor.[16][18][19] It is about 0.2% to 1% as potent as an estrogen as estradiol.[18]
See also
editReferences
edit- ^ "4-Hydroxybenzoic acid" (PDF). International Programme on Chemical Safety (IPCS). Archived from the original (PDF) on 24 September 2015. Retrieved 10 January 2015.
- ^ Juteau, P.; Côté, V.; Duckett, M.-F.; Beaudet, R.; Lépine, F.; Villemur, R.; Bisaillon, J.-G. (January 2005). "Cryptanaerobacter phenolicus gen. nov., sp. nov., an anaerobe that transforms phenol into benzoate via 4-hydroxybenzoate". International Journal of Systematic and Evolutionary Microbiology. 55 (1): 245–250. doi:10.1099/ijs.0.02914-0. PMID 15653882.
- ^ Dey, G.; Chakraborty, M.; Mitra, A. (April 2005). "Profiling C6–C3 and C6–C1 phenolic metabolites in Cocos nucifera". Journal of Plant Physiology. 162 (4): 375–381. doi:10.1016/j.jplph.2004.08.006. PMID 15900879.
- ^ Pietta, P. G.; Simonetti, P.; Gardana, C.; Brusamolino, A.; Morazzoni, P.; Bombardelli, E. (1998). "Catechin metabolites after intake of green tea infusions". BioFactors. 8 (1–2): 111–118. doi:10.1002/biof.5520080119. PMID 9699018. S2CID 37684286.
- ^ Tian, R.-R.; Pan, Q.-H.; Zhan, J.-C.; Li, J.-M.; Wan, S.-B.; Zhang, Q.-H.; Huang, W.-D. (2009). "Comparison of phenolic acids and flavan-3-ols during wine fermentation of grapes with different harvest times". Molecules. 14 (2): 827–838. doi:10.3390/molecules14020827. PMC 6253884. PMID 19255542.
- ^ Goulas, V.; Stylos, E.; Chatziathanasiadou, M. V.; Mavromoustakos, T.; Tzakos, A. G. (2016). "Functional Components of Carob Fruit: Linking the Chemical and Biological Space". International Journal of Molecular Sciences. 17 (11): 1875. doi:10.3390/ijms17111875. PMC 5133875. PMID 27834921.
- ^ Pacheco Palencia, L. A.; Mertens-Talcott, S.; Talcott, S. T. (June 2008). "Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Açaí (Euterpe oleracea Mart.)". Journal of Agricultural and Food Chemistry. 56 (12): 4631–4636. doi:10.1021/jf800161u. PMID 18522407.
- ^ Münzenberger, B.; Heilemann, J.; Strack, D.; Kottke, I.; Oberwinkler, F. (1990). "Phenolics of mycorrhizas and non-mycorrhizal roots of Norway spruce". Planta. 182 (1): 142–148. doi:10.1007/BF00239996. PMID 24197010. S2CID 43504838.
- ^ Hoberg, E.; Meier, B.; Sticher, O. (September 2000). "An analytical high performance liquid chromatographic method for the determination of agnuside and p-hydroxybenzoic acid contents in Agni-casti fructose". Phytochemical Analysis. 11 (5): 327–329. Bibcode:2000PChAn..11..327H. doi:10.1002/1099-1565(200009/10)11:5<327::AID-PCA523>3.0.CO;2-0.
- ^ Acosta, Manuel Jesús; Vazquez Fonseca, Luis; Desbats, Maria Andrea; Cerqua, Cristina; Zordan, Roberta; Trevisson, Eva; Salviati, Leonardo (2016). "Coenzyme Q biosynthesis in health and disease". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1857 (8): 1079–1085. doi:10.1016/j.bbabio.2016.03.036. PMID 27060254.
- ^ Edwin Ritzer and Rudolf Sundermann "Hydroxycarboxylic Acids, Aromatic" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_519
- ^ Buehler, C. A.; Cate, W. E. (1943). "p-Hydroxybenzoic acid". Organic Syntheses; Collected Volumes, vol. 2, p. 341.
- ^ Charles Fishman, Dan Winters (2016-04-11). "This Expandable Structure Could Become the Future of Living in Space". Smithsonian Magazine. Retrieved 2020-12-07.
- ^ Lewis, R. J., ed. (1996). Sax's Dangerous Properties of Industrial Materials. Vol. 1–3 (9th ed.). New York, NY: Van Nostrand Reinhold. p. 2897.
- ^ a b Khetan, S. K. (23 May 2014). Endocrine Disruptors in the Environment. Wiley. p. 109. ISBN 978-1-118-89115-5.
- ^ a b c Pugazhendhi, D.; Pope, G. S.; Darbre, P. D. (2005). "Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines". Journal of Applied Toxicology. 25 (4): 301–309. doi:10.1002/jat.1066. PMID 16021681. S2CID 12342018.
- ^ a b Gabriel, J. (April 2013). Holistic Beauty from the Inside Out: Your Complete Guide to Natural Health, Nutrition, and Skincare. Seven Stories Press. p. 31. ISBN 978-1-60980-462-6.
- ^ a b Lemini, C.; Silva, G.; Timossi, C.; Luque, D.; Valverde, A.; González Martínez, M.; Hernández, A.; Rubio Póo, C.; Chávez Lara, B.; Valenzuela, F. (1997). "Estrogenic effects of p-hydroxybenzoic acid in CD1 mice". Environmental Research. 75 (2): 130–134. Bibcode:1997ER.....75..130L. doi:10.1006/enrs.1997.3782. PMID 9417843.
- ^ OECD (November 2004). OECD Guidelines for the Testing of Chemicals / OECD Series on Testing and Assessment Detailed Background Review of the Uterotrophic Bioassay. OECD Publishing. p. 183. ISBN 978-92-64-07885-7.
