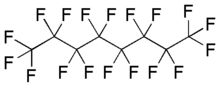
Perfluorooctane, also known as octadecafluorooctane, is a fluorocarbon liquid—a perfluorinated derivative of the hydrocarbon octane. It can be a good substitute for insulating oil in high voltage electronics. In addition to heat transfer applications, it has also been used as a breathable fluid in partial liquid ventilation.[2]
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Octadecafluorooctane | |
| Other names
PF5080
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.005.637 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8F18 | |
| Molar mass | 438.06 g/mol |
| Appearance | Clear, colorless liquid |
| Density | 1.766 g/mL |
| Melting point | −25 °C (−13 °F; 248 K) |
| Boiling point | 103 to 104 °C (217 to 219 °F; 376 to 377 K) |
| 10 ppm | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
None |
| Flash point | None |
| None | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Production
editPerfluorooctane can be produced by the Fowler process or by electrochemical fluorination.
Fowler process
editThe Fowler process involves moderating the action of elemental fluorine with cobalt fluoride in the gas phase from octane.
Electrochemical fluorination
editElectrolysis in hydrogen fluoride of nonanoic acid will produce both perfluorononanoic acid and perfluorooctane. Perfluorooctane manufactured this way is marketed under the name PF5080 (or FC77) by 3M as part of their Fluorinert range of heat transfer fluids.[3]
Applications
editPerfluorooctane is chemically inert, but has useful physical properties, leading to its employment in diverse areas:
References
edit- ^ Perfluorooctane at Sigma-Aldrich
- ^ H. Proquitté; M. Rüdiger; R. R. Wauer & G. Schmalisch (2003). "Breathing gas perfluorocarbon measurements using an absorber filled with zeolites". British Journal of Anaesthesia. 91 (5): 736–8. doi:10.1093/bja/aeg247. PMID 14570799.
- ^ "3M Performance Fluid PF-5080". 3M.
- ^ "Tradeline Medical - Buy Medical Grade Perfluorooctane". tradelinemedical.net. Archived from the original on 2014-05-20.
- ^ Claes C, Worst J, Zivojnovic R (1992). "Retinal detachment surgery following implantation of a keratoprosthesis. A case report". Bulletin de la Société Belge d'Ophtalmologie. 243: 167–169. PMID 1302147.