Phentermine, sold under the brand name Adipex-P among others, is a medication used together with diet and exercise to treat obesity.[3] It is available by itself or as the combination phentermine/topiramate.[6]
 | |||
| |||
| Clinical data | |||
|---|---|---|---|
| Trade names | Adipex-P, Ionamin, Suprenza, others | ||
| Other names | α-methyl-amphetamine α,α-dimethylphenethylamine | ||
| AHFS/Drugs.com | Monograph | ||
| MedlinePlus | a682187 | ||
| Pregnancy category |
| ||
| Dependence liability | Physical: Not typical Psychological: Moderate[1] | ||
| Addiction liability | Low[2] | ||
| Routes of administration | By mouth | ||
| Drug class | Appetite suppressant[3] | ||
| ATC code | |||
| Legal status | |||
| Legal status |
| ||
| Pharmacokinetic data | |||
| Bioavailability | High (almost complete)[5] | ||
| Protein binding | Approximately 96.3% | ||
| Metabolism | Liver[5] | ||
| Elimination half-life | 25 hours, urinary pH-dependent[5] | ||
| Excretion | Kidney (62–85% unchanged)[5] | ||
| Identifiers | |||
| |||
| CAS Number | |||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEBI | |||
| ChEMBL | |||
| CompTox Dashboard (EPA) | |||
| ECHA InfoCard | 100.004.112 | ||
| Chemical and physical data | |||
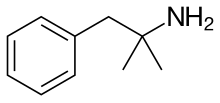
| Formula | C10H15N | ||
| Molar mass | 149.237 g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
| (verify) | |||
Common side effects include a fast heart beat, high blood pressure, trouble sleeping, dizziness, and restlessness.[3] Serious side effects may include abuse, but do not include pulmonary hypertension or valvular heart disease, as the latter were caused by the fenfluramine component of the fen-phen drug combination.[3] It works mainly as an appetite suppressant, likely as a result of being a central nervous system (CNS) stimulant.[3] Chemically, phentermine is a substituted amphetamine.[7]
Phentermine was approved for medical use in the United States in 1959.[3] It is available as a generic medication.[3] In 2022, it was the 149th most commonly prescribed medication in the United States, with more than 3 million prescriptions.[8][9] Phentermine was withdrawn from the market in the United Kingdom in 2000, while the combination medication fen-phen, of which it was a part, was withdrawn from the market in 1997 due to side effects of fenfluramine.[10]
Medical uses
editPhentermine is used for a short period of time to promote weight loss, if exercise and calorie reduction are not sufficient, and in addition to exercise and calorie reduction.[5][11]
Phentermine is approved for up to 12 weeks of use and most weight loss occurs in the first weeks.[11] However, significant loss continues through the sixth month and has been shown to continue at a slower rate through the ninth month.[12]
Contraindications
editUse is not recommended during pregnancy or breastfeeding,[13] or with selective serotonin reuptake inhibitors (SSRIs) or monoamine oxidase inhibitors (MAOIs).[3]
Phentermine is contraindicated for users who:[5][11]
- have a history of drug abuse
- are allergic to sympathomimetic amine drugs
- are taking a monoamine oxidase inhibitor (MAOI) or have taken one within the last 14 days
- have cardiovascular disease, hyperthyroidism, or glaucoma
- are pregnant, planning to become pregnant, or breastfeeding.
Adverse effects
editTolerance usually occurs; however, risks of dependence and addiction are considered negligible.[12][14] People taking phentermine may be impaired when driving or operating machinery.[11] Consumption of alcohol with phentermine may produce adverse effects.[11]
There is currently no evidence regarding whether or not phentermine is safe for pregnant women.[5][11]
Other adverse effects include:[5][11]
- Cardiovascular effects like palpitations, tachycardia, high blood pressure, precordial pain; rare cases of stroke, angina, myocardial infarction, cardiac failure and cardiac arrest have been reported.
- Central nervous system effects like overstimulation, restlessness, nervousness, insomnia, tremor, dizziness and headache; there are rare reports of euphoria followed by fatigue and depression, and very rarely, psychotic episodes and hallucinations.
- Gastrointestinal effects include nausea, vomiting, dry mouth, cramps, unpleasant taste, diarrhea, and constipation.
- Other adverse effects include trouble urinating, rash, impotence, changes in libido, and facial swelling.
Interactions
editPhentermine may decrease the effect of drugs like clonidine, methyldopa, and guanethidine. Drugs to treat hypothyroidism may increase the effect of phentermine.[11]
Mechanism of action
editPhentermine has some similarity in its pharmacodynamics with its parent compound, amphetamine, as they both are TAAR1 agonists,[15] where the activation of TAAR1 in monoamine neurons facilitates the efflux, or release into the synapse, of these neurochemicals. At clinically relevant doses, phentermine primarily acts as a releasing agent of norepinephrine in neurons, although, to a lesser extent, it releases dopamine and serotonin into synapses as well.[14][16] Phentermine may also trigger the release of monoamines from VMAT2, which is a common pharmacodynamic effect among substituted amphetamines. The primary mechanism of phentermine's action in treating obesity is the reduction of hunger perception, which is a cognitive process mediated primarily through several nuclei within the hypothalamus (in particular, the lateral hypothalamic nucleus, arcuate nucleus, and ventromedial nucleus). Outside the brain, phentermine releases norepinephrine and epinephrine – also known as noradrenaline and adrenaline respectively – causing fat cells to break down stored fat as well.
History
editIn 1959, phentermine first received approval from the US Food and Drug Administration (FDA) as an appetite suppressant.[17] Eventually a hydrochloride salt and a resin form became available.[17]
Phentermine was marketed with fenfluramine or dexfenfluramine as a combination appetite suppressant and fat burning agent under the popular name fen-phen.[18] In 1997, after 24 cases of heart valve disease in fen-phen users, fenfluramine and dexfenfluramine were voluntarily taken off the market at the request of the FDA.[19] Studies later showed nearly 30% of people taking fenfluramine or dexfenfluramine for up to 24 months had abnormal valve findings.[20]
Phentermine is still available by itself in most countries, including the US.[17] However, because it is similar to amphetamine, it is classified as a controlled substance in many countries. Internationally, phentermine is a schedule IV drug under the Convention on Psychotropic Substances.[21] In the United States, it is classified as a Schedule IV controlled substance under the Controlled Substances Act. In contrast, amphetamine preparations are classified as Schedule II controlled substances.[22]
A company called Vivus developed a combination drug, phentermine/topiramate that it originally called Qnexa and then called Qsymia, which was invented and used off-label by Thomas Najarian, who opened a weight-clinic in Los Osos, California in 2001; Najarian had previously worked at Interneuron Pharmaceuticals, which had developed one of the fen-phen drugs previously withdrawn from the market.[23] The FDA rejected the combination drug in 2010 due to concerns over its safety.[23] In 2012 the FDA approved it after Vivus re-applied with further safety data.[24] At the time, one obesity specialist estimated that around 70% of his colleagues were already prescribing the combination off-label.[23]
Chemistry
editPhentermine is a substituted amphetamine which has a methyl group on amphetamine's alpha carbon.[7] It is a positional isomer of methamphetamine and other methylamphetamines. The molecular formula of phentermine is C10H15N.
Names
editThe term ‘phentermine' is contracted from phenyl-tertiary-butyl amine.
It is marketed under many brand names and formulations worldwide, including Acxion, Adipex, Adipex-P, Duromine, Elvenir, Fastin, Ionamin, Lomaira (phentermine hydrochloride), Panbesy, Qsymia (phentermine and topiramate), Razin, Redusa, Sentis, Suprenza, and Terfamex.[25]
References
edit- ^ Tarascon Pocket Pharmacopoeia 2017 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. 2016. p. 7. ISBN 9781284118971.
- ^ Sadock BJ, Sadock VA (2010). Kaplan and Sadock's Pocket Handbook of Clinical Psychiatry. Lippincott Williams & Wilkins. p. 435. ISBN 9781605472645.
- ^ a b c d e f g h "Phentermine Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 13 April 2019.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ a b c d e f g h "METERMINE (Phentermine)" (PDF). TGA eBusiness Services. iNova Pharmaceuticals (Australia) Pty Limited. 22 July 2013. Retrieved 16 November 2013.
- ^ "Phentermine and topiramate Uses, Side Effects & Warnings". Drugs.com. Retrieved 13 April 2019.
- ^ a b Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (July 2012). "Biosynthesis of amphetamine analogs in plants". Trends in Plant Science. 17 (7): 404–412. Bibcode:2012TPS....17..404H. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Phentermine Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ Bagchi D, Preuss HG (2012). Obesity: Epidemiology, Pathophysiology, and Prevention (Second ed.). CRC Press. p. 314. ISBN 9781439854259.
- ^ a b c d e f g h "Phentermine label at FDA" (Last updated: January 2012). FDA. Retrieved 13 October 2016.
- ^ a b Glazer G (August 2001). "Long-term pharmacotherapy of obesity 2000: a review of efficacy and safety". Archives of Internal Medicine. 161 (15): 1814–1824. doi:10.1001/archinte.161.15.1814. PMID 11493122.
- ^ "Phentermine Use During Pregnancy". Drugs.com. Retrieved 13 April 2019.
- ^ a b Haslam D (March 2016). "Weight management in obesity - past and present". International Journal of Clinical Practice. 70 (3): 206–217. doi:10.1111/ijcp.12771. PMC 4832440. PMID 26811245.
- ^ Barak LS, Salahpour A, Zhang X, Masri B, Sotnikova TD, Ramsey AJ, et al. (September 2008). "Pharmacological characterization of membrane-expressed human trace amine-associated receptor 1 (TAAR1) by a bioluminescence resonance energy transfer cAMP biosensor". Molecular Pharmacology. 74 (3): 585–594. doi:10.1124/mol.108.048884. PMC 3766527. PMID 18524885.
we confirmed agonistic activity at human TAAR1 of several other compounds, including the trace amines octopamine and tryptamine, the amphetamine derivatives l-amphetamine, d-methamphetamine, (+)-MDMA, and phentermine, and the catecholamine metabolites 3-MT and 4-MT (Bunzow et al., 2001; Lindemann and Hoener, 2005; Reese et al., 2007; Wainscott et al., 2007; Wolinsky et al., 2007; Xie and Miller, 2007; Xie et al., 2007).
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. (January 2001). "Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin". Synapse. 39 (1): 32–41. doi:10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. PMID 11071707. S2CID 15573624.
- ^ a b c Ryan DA, Bray GA (2014). "Sibutramine, Phentermine, and Diethylproprion: Sympathomimetic Drugs in the Management of Obesity". In Bray GA, Bouchard C (eds.). Handbook of Obesity - Volume 2 Clinical Applications (Fourth ed.). Hoboken: Taylor and Francis. p. 234. ISBN 9781841849829.
- ^ Kolata G (23 September 1997). "How Fen-Phen, A Diet 'Miracle,' Rose and Fell". New York Times. NY, NY, USA.
- ^ "FDA Announces Withdrawal Fenfluramine and Dexfenfluramine (Fen-Phen)". Fda.gov. Retrieved 12 July 2013.
- ^ Weigle DS (June 2003). "Pharmacological therapy of obesity: past, present, and future". The Journal of Clinical Endocrinology and Metabolism. 88 (6): 2462–2469. doi:10.1210/jc.2003-030151. PMID 12788841.
- ^ Convention on Psychotropic Substances Archived 14 March 2014 at the Wayback Machine
- ^ Rueda-Clausen CF, Padwal RS, Sharma AM (August 2013). "New pharmacological approaches for obesity management". Nature Reviews. Endocrinology. 9 (8): 467–478. doi:10.1038/nrendo.2013.113. PMID 23752772. S2CID 20072687.
- ^ a b c Pollack A (16 February 2012). "Diet Treatment, Already in Use, to Get F.D.A. Review". The New York Times.
- ^ "FDA approves weight-management drug Qsymia". FDA. 17 July 2012.
- ^ "International brands for phentermine". Drugs.com. Retrieved 13 October 2016.
External links
edit- "Phentermine". International Programme on Chemical Safety (IPCS). the World Health Organization (WHO).

