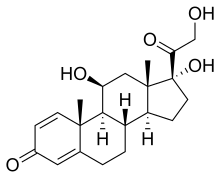
Prednisolone is a corticosteroid, a steroid hormone used to treat certain types of allergies, inflammatory conditions, autoimmune disorders, and cancers.[5][6] Some of these conditions include adrenocortical insufficiency, high blood calcium, rheumatoid arthritis, dermatitis, eye inflammation, asthma, and multiple sclerosis.[6] It can be taken by mouth, injected into a vein, used topically as a skin cream, or as eye drops.[7][8][6] It differs from the similarly named prednisone in having a hydroxyl at the 11th carbon instead of a ketone.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Orapred, PediaPred, Millipred, others |
| Other names | 11,17-Dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a615042 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous, topical, ophthalmic |
| Drug class | Glucocorticoid |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 2–3.5 hours[2][3][4] |
| Excretion | urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.020 |
| Chemical and physical data | |
| Formula | C21H28O5 |
| Molar mass | 360.450 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Common side effects with short-term use include nausea, difficulty concentrating, insomnia, increased appetite, and fatigue.[5] More severe side effects include psychiatric problems, which may occur in about 5% of people.[9] Common side effects with long-term use include bone loss, weakness, yeast infections, and easy bruising.[6] While short-term use in the later part of pregnancy is safe, long-term use or use in early pregnancy is occasionally associated with harm to the baby.[1] It is a glucocorticoid made from hydrocortisone (cortisol).[10]
Prednisolone was discovered and approved for medical use in 1955.[10] It is on the World Health Organization's List of Essential Medicines.[11] It is available as a generic drug.[6] In 2022, it was the 136th most commonly prescribed medication in the United States, with more than 4 million prescriptions.[12][13]
Medical uses
editWhen used in low doses, corticosteroids serve as an anti-inflammatory agent. At higher doses, they are considered as immunosuppressants.[14] Corticosteroids inhibit the inflammatory response to a variety of inciting agents and, it is presumed, delay or slow healing.[15] They inhibit edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation.[16]
Systemic use
editPrednisolone is a corticosteroid drug with predominant glucocorticoid and low mineralocorticoid activity, making it useful for the treatment of a wide range of inflammatory and autoimmune conditions[17] such as asthma,[18] uveitis, pyoderma gangrenosum, rheumatoid arthritis, urticaria,[19] angioedema,[19] ulcerative colitis, pericarditis, temporal arteritis, Crohn's disease, Bell's palsy, multiple sclerosis,[20] cluster headaches, vasculitis, acute lymphoblastic leukemia, autoimmune hepatitis,[21] lupus, Kawasaki disease,[22] dermatomyositis,[7] post-myocardial infarction syndrome,[23] and sarcoidosis.[24]
Prednisolone can also be used for allergic reactions ranging from seasonal allergies to drug allergic reactions.[25]
Prednisolone can also be used as an immunosuppressant for organ transplants.[7][26]
Prednisolone in lower doses can be used in cases of adrenal insufficiency due to Addison's disease.[27][28]
Topical use
editOphthalmology
Topical prednisolone is mainly used in the ophthalmic pathway as eye drops in numerous eye conditions,[29] including corneal injuries caused by chemicals, burns, and alien objects, inflammation of the eyes, mild to moderate non-infectious allergies, disorders of the eyelid, conjunctiva or sclera, ocular inflammation caused by operation and optic neuritis.[30][31] Some side effects include glaucoma, blurred vision, eye discomfort, impaired recovery of injured site, scarring of the optic nerve, cataracts, and urticaria.[31] However, their prevalence is not known.
Prednisolone eye drops are contraindicated in individuals who develop hypersensitivity reactions against prednisolone,[29] or individuals with the current conditions, such as tuberculosis of the eye, shingles affecting the eye, raised intraocular pressure, and eye infection caused by fungus.[32]
Prednisolone acetate ophthalmic suspension (eye drops) is prepared as a sterile ophthalmic suspension and used to reduce swelling, redness, itching, and allergic reactions affecting the eye.[33][8][34] It has been explored as a treatment option for bacterial keratitis.[35]
Prednisolone eye drops are used in conjunctivitis caused by allergies and bacteria, marginal keratitis, uveitis, endophthalmitis, which is an infection of the eye involving the aqueous humor, Graves' ophthalmopathy, herpes zoster ocular infection, inflammation of the eye after surgery, and corneal injuries caused by chemicals, radiation, thermal burns, or penetration of foreign objects.[30] It is also used in the prevention of myringosclerosis,[36] herpes simplex stromal keratitis.[37] Topical prednisolone can also be used after procedures such as Laser Peripheral Iridotomy for patients with primary angle-closure suspects (PACS) to control inflammations.[38]
Ear drops
In addition, topical prednisolone can also be administered as ear drops.[39]
| Eye drops | Ear drops |
|---|---|
| Prednisolone sodium phosphate ophthalmic solution[29] | Prednisolone sodium phosphate 0.5% eye/ ear drops[39] |
| Pred forte ophthalmic suspension (prednisolone acetate ophthalmic suspension)[33] | |
| Suspension (prednisolone mild ophthalmic)[29] | |
| Suspension (prednisolone acetate ophthalmic)[29] |
Adverse effects
editAdverse reactions from the use of prednisolone include:[40][7]
- Increased appetite, weight gain, nausea, and malaise
- Increased risk of infection
- Cardiovascular events
- Dermatological effects including reddening of face, bruising/skin discoloration, impaired wound healing, skin atrophy, skin rash, edema, and abnormal hair growth
- Hyperglycemia; patients with diabetes may need increased insulin or diabetic therapies
- Menstrual abnormalities
- Lower response to hormones, especially during stressful instances such as surgery or illness
- Change in electrolytes: rise in blood pressure, increased sodium and low potassium, leading to alkalosis
- Gastrointestinal system effects: swelling of stomach lining, reversible increase in liver enzymes, and risk of stomach ulcers
- Muscular and skeletal abnormalities, such as muscle weakness/muscle loss, osteoporosis (see steroid-induced osteoporosis), long bone fractures, tendon rupture, and back fractures
- Neurological effects, including involuntary movements (convulsions), headaches, and vertigo
- Psychosocial behavioral and emotional disturbances[41] with aggression being one of the most common cognitive symptoms, especially with oral use.[42]
- Nasal septum perforation and bowel perforation (in some pathologic conditions).[43][44]
Discontinuing prednisolone after long-term or high-dose use can lead to adrenal insufficiency.[41]
Pregnancy and breastfeeding
editAlthough there are no major human studies of prednisolone use in pregnant women, studies in several animals show that it may cause birth defects including increased likelihood of cleft palate.
Prednisolone is found in breast milk of mothers taking prednisolone.[41]
Local adverse effects in the eye
editWhen used topically on the eye, the following are potential side-effects:
- Cataracts: Extended usage of corticosteroids may cause clouding at the back of the lens, also known as posterior subcapsular cataract. This type of cataract reduces the path of light from reaching the eye, which interferes with a person's reading vision. Consumption of prednisolone eye drops post surgery may also retard the healing process.[29]
- Corneal thinning: When corticosteroids are used in the long term, corneal and scleral thinning is also one of its consequences. When not ceased, thinning may ultimately lead to perforation of the cornea.[29]
- Glaucoma: Elongated use of corticosteroids has a chance of causing a raised intraocular pressure (IOP), injuring the optic nerve and weakening of visual awareness. Corticosteroids should be used cautiously in patients with concomitant conditions of glaucoma. Doctors track patients' IOP if they are using corticosteroid eye drops for more than 103 days.[29]
Pharmacology
editPharmacodynamics
editAs a glucocorticoid, the lipophilic structure of prednisolone allows for easy passage through the cell membrane where it then binds to its respective glucocorticoid receptor (GCR) located in the cytoplasm. Upon binding, formation of the GC/GCR complex causes dissociation of chaperone proteins from the glucocorticoid receptor enabling the GC/GCR complex to translocate inside the nucleus.[45] This process occurs within 20 minutes of binding. Once inside the nucleus, the homodimer GC/GCR complex binds to specific DNA binding-sites known as glucocorticoid response elements (GREs) resulting in gene expression or inhibition. Complex binding to positive GREs leads to synthesis of anti-inflammatory proteins while binding to negative GREs blocks the transcription of inflammatory genes.[46] They inhibit the release of signals that promote inflammation such as nuclear factor-Kappa B (NF-κB), Activator protein 1 (AP-1), nuclear factor of activated T-cells (NFAT), and stimulate anti-inflammatory signals such as the interleukin-10 gene.[47][14] All of them will collectively cause a sequence of events, including the inhibition of prostaglandin synthesis and additional inflammatory mediators. Glucocorticoids also inhibit neutrophil cell death and demargination. As well as phospholipase A2, which in turn lessens arachidonic acid derivative genesis.[48]
Pharmacokinetics
editPrednisolone has a relatively short half-life, ranging 2–4 hours. It also has a large therapeutic window, considering the dosage required to produce a therapeutic effect is a few times higher than what the body naturally produces.[14]
Prednisolone is 70–90% plasma protein bound, it binds to proteins such as albumin.[14]
Both prednisolone phosphate and prednisolone acetate go through ester hydrolysis in the body to form prednisolone. It subsequently undergoes the usual metabolism of prednisolone. Concomitant use of prednisolone and strong CYP3A4 inhibitors such as ketoconazole is shown to cause a rise in plasma prednisolone concentrations by about 50% owing to a diminished clearance.[47]
Prednisolone predominantly undergoes kidney elimination and is excreted in the urine as sulphate and metabolites of glucuronide conjugate.[14]
Prednisone
editPrednisone is a prodrug that is activated in the liver. When it enters the body, prednisone is triggered by the liver and body chemicals to turn into its active form, prednisolone.[49]
Chemistry
editPrednisolone is a synthetic pregnane corticosteroid closely related to its cognate prednisone, having identical structure save for two fewer hydrogens near C11. It is also known as δ1-cortisol, δ1-hydrocortisone, 1,2-dehydrocortisol, or 1,2-dehydrohydrocortisone, as well as 11β,17α,21-trihydroxypregna-1,4-diene-3,20-dione.[50][51]
Interactions
editCo-administration of prednisolone eye drops with ophthalmic nonsteroidal anti-inflammatory drugs (NSAIDs) may perhaps exacerbate its effects, causing unwanted side effects such as toxicity.[clarification needed] The wound healing process may also be hindered.[52]
Drug interactions of prednisolone include other immunosuppressants like azathioprine or ciclosporin, antiplatelet drugs like clopidogrel, anticoagulants like dabigatran or warfarin, or NSAIDs such as aspirin, celecoxib, or ibuprofen.[53]
Contraindications
editThis section is empty. You can help by adding to it. (October 2023) |
Special populations
editChildren
editProlonged use of prednisolone eye drops in children may lead to a raised intraocular pressure. While this phenomenon is dose dependent, it is shown to have a greater effect especially in children under 6 years of age.[29]
Pregnancy and breastfeeding
editResearches on animal reproduction have indicated that there is a trace of teratogenicity when doses are reduced by 10 times of the human recommended dose.[54] There is no sufficient information on human pregnancy at this moment. Use is only recommended when the potential benefits outweigh the potential risks of the pregnant mother and the fetus.[54]
Prednisolone when delivered systemically can be found in the mother's breast milk, however, there is no data provided for the extent of prednisolone found in the system after administering eye drops.[29][54] However, the presence of corticosteroids is recorded when they are administered systemically, and it could possibly affect the fetus' growth.[54] Therefore, use of prednisolone during breastfeeding is not advocated.[54]
Society and culture
editDosage forms
editPrednisolone is supplied as oral liquid, oral suspension, oral syrup, oral tablet, and oral disintegrating tablet. It may be a generic medication or supplied as brands Flo-Pred (prednisolone acetate oral suspension),[40] Millipred (oral tablets),[25] Orapred (prednisolone sodium phosphate oral dissolving tablets),[7] Pediapred (prednisolone sodium phosphate oral solution),[41] Veripred 20, Prelone, Hydeltra-T.B.A., Hydeltrasol, Key-Pred, Cotolone, Predicort, Medicort, Predcor, Bubbli-Pred, Omnipred (prednisolone acetate ophthalmic suspension),[8] Pred Mild,[34] Pred Forte,[33] and others.[55]
Athletics
editAs a glucocorticosteroid, unauthorized or ad hoc use of prednisolone during competition via oral, intravenous, intramuscular or rectal routes is banned under World Anti-Doping Agency (WADA) anti-doping rules.[56]
Veterinary uses
editPrednisolone is used in the treatment of inflammatory and allergic conditions in cats, dogs, horses, small mammals such as ferrets, birds, and reptiles.[57][58] Its usage in treating inflammation, immune-mediated disease, Addison's disease, and neoplasia is often considered off-label use. Many drugs are commonly prescribed for off label use in veterinary medicine."[59] Studies in ruminating species, such as alpacas, have shown that oral administration of the drug is associated with a reduced bioavailability compared to intravenous administration; however, levels that are therapeutic in other species can be achieved with oral administration in alpacas.[60]
It is used in a broad spectrum of diseases, for example, inflammation of scleral tissues, cornea, conjunctiva in dogs.[57] In horses, prednisolone acetate suspensions are priorly used to treat inflammation in the middle layer of the eye, also known as anterior uveitis and equine recurrent uveitis (ERU), which is the leading cause of visual impairment in horses.[58] Prednisolone acetate eye drops are not to be used in other animals such as birds.[57]
Prednisolone acetate eye drops are also prescribed to the dogs and cats to lessen swelling, redness, burning and pain sensations after surgeries of the eye.[57]
Cats with conjunctivitis usually are required to avoid using ophthalmic preparations of corticosteroids and its derivatives. The most typical infections are caused by herpes virus.[58]
References
edit- ^ a b "Prednisolone Use During Pregnancy". Drugs. 16 January 2000. Archived from the original on 21 December 2016. Retrieved 9 March 2020.
- ^ Pickup ME (1979). "Clinical pharmacokinetics of prednisone and prednisolone". Clinical Pharmacokinetics. 4 (2): 111–28. doi:10.2165/00003088-197904020-00004. PMID 378499. S2CID 12218704.
- ^ Bergrem H, Grøttum P, Rugstad HE (1983). "Pharmacokinetics and protein binding of prednisolone after oral and intravenous administration". European Journal of Clinical Pharmacology. 24 (3): 415–9. doi:10.1007/BF00610064. PMID 6861855. S2CID 33189235.
- ^ Bashar T, Apu MN, Mostaid MS, Islam MS, Hasnat A (2018). "Pharmacokinetics and Bioavailability Study of a Prednisolone Tablet as a Single Oral Dose in Bangladeshi Healthy Volunteers". Dose-response. 16 (3). doi:10.1177/1559325818783932. PMC 6073839. PMID 30083083.
- ^ a b Stuart MC, Kouimtzi M, Hill SR, eds. (2009). WHO Model Formulary 2008. World Health Organization. pp. 53–54. hdl:10665/44053. ISBN 978-924154765-9.
- ^ a b c d e "Prednisolone". The American Society of Health-System Pharmacists. Archived from the original on 23 December 2016. Retrieved 8 December 2016.
- ^ a b c d e "Orapred ODT- prednisolone sodium phosphate tablet, orally disintegrating". DailyMed. 11 September 2019. Archived from the original on 12 May 2021. Retrieved 9 March 2020.
- ^ a b c "Omnipred- prednisolone acetate suspension". DailyMed. 9 September 2019. Archived from the original on 13 May 2021. Retrieved 9 March 2020.
- ^ "Pevanti 10mg Tablets – Summary of Product Characteristics (SPC) – (eMC)". Medicines. UK. 1 December 2014. Archived from the original on 20 December 2016. Retrieved 13 December 2016.
- ^ a b Kim KW, Roh JK, Wee HJ, Kim C (2016). Cancer Drug Discovery: Science and History. Springer. p. 169. ISBN 978-940240844-7. Archived from the original on 10 September 2017.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Prednisolone Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ a b c d e "Prednisolone phosphate". go.drugbank.com. Archived from the original on 25 February 2023. Retrieved 16 March 2022.
- ^ Schwiebert LM, Beck LA, Stellato C, Bickel CA, Bochner BS, Schleimer RP, Schwiebert LA (January 1996). "Glucocorticosteroid inhibition of cytokine production: relevance to antiallergic actions". The Journal of Allergy and Clinical Immunology. 97 (1 Pt 2): 143–52. doi:10.1016/s0091-6749(96)80214-4. PMID 8568145. S2CID 27261595.
- ^ Lee SH (November 2015). "Mechanisms of Glucocorticoid Action in Chronic Rhinosinusitis". Allergy, Asthma & Immunology Research. 7 (6): 534–7. doi:10.4168/aair.2015.7.6.534. PMC 4605925. PMID 26333699.
- ^ Czock D, Keller F, Rasche FM, Häussler U (2005). "Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids". Clinical Pharmacokinetics. 44 (1): 61–98. doi:10.2165/00003088-200544010-00003. PMID 15634032. S2CID 24458998.
- ^ Fiel SB, Vincken W (June 2006). "Systemic corticosteroid therapy for acute asthma exacerbations". The Journal of Asthma. 43 (5): 321–31. doi:10.1080/02770900600567163. PMID 16801135. S2CID 24656857.
- ^ a b Spickett G (2014). "Urticaria and angioedema". J R Coll Physicians Edinb. 44 (1): 50–4. doi:10.4997/JRCPE.2014.112. PMID 24995449.
- ^ Thrower BW (January 2009). "Relapse management in multiple sclerosis". The Neurologist. 15 (1): 1–5. doi:10.1097/NRL.0b013e31817acf1a. PMID 19131851. S2CID 10687818.
- ^ Lambrou GI, Vlahopoulos S, Papathanasiou C, Papanikolaou M, Karpusas M, Zoumakis E, Tzortzatou-Stathopoulou F (December 2009). "Prednisolone exerts late mitogenic and biphasic effects on resistant acute lymphoblastic leukemia cells: Relation to early gene expression". Leukemia Research. 33 (12): 1684–95. doi:10.1016/j.leukres.2009.04.018. PMID 19450877.
- ^ Miura M, Tamame T, Naganuma T, Chinen S, Matsuoka M, Ohki H (October 2011). "Steroid pulse therapy for Kawasaki disease unresponsive to additional immunoglobulin therapy". Paediatrics & Child Health. 16 (8): 479–84. doi:10.1093/pch/16.8.479. PMC 3202387. PMID 23024586.
- ^ "Prednisolone 5mg Tablets - Summary of Product Characteristics (SmPC) - (emc)". Medicines. UK. Archived from the original on 27 November 2022. Retrieved 27 November 2022.
- ^ Gera K, Gupta N, Ahuja A, Shah A (April 2014). "Acute alveolar sarcoidosis presenting with hypoxaemic respiratory failure". BMJ Case Reports. 2014: bcr2013202247. doi:10.1136/bcr-2013-202247. PMC 4024548. PMID 24789154.
- ^ a b "Millipred- prednisolone tablet". DailyMed. NIH. 8 April 2019. Archived from the original on 9 March 2020. Retrieved 9 March 2020.
- ^ Vethe NT, Midtvedt K, Asberg A, Amundsen R, Bergan S (October 2011). "[Drug interactions and immunosuppression in organ transplant recipients]". Tidsskrift for den Norske Laegeforening. 131 (20): 2000–3. doi:10.4045/tidsskr.11.0138. PMID 22016125.
- ^ Husebye ES, Allolio B, Arlt W, Badenhoop K, Bensing S, Betterle C, Falorni A, Gan EH, Hulting AL, Kasperlik-Zaluska A, Kämpe O, Løvås K, Meyer G, Pearce SH (February 2014). "Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency". Journal of Internal Medicine. 275 (2): 104–15. doi:10.1111/joim.12162. PMID 24330030. S2CID 206117017.
- ^ AMA Department of Drugs: AMA Drug Evaluations (6th ed.). Chicago, IL: American Medical Association. 1986.
- ^ a b c d e f g h i j "Prednisolone (ophthalmic): Drug information".
- ^ a b "Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 13e > Ocular Pharmacy". AccessPharmacy. Archived from the original on 16 March 2022. Retrieved 16 March 2022.
- ^ a b "Prednisolone (Ophthalmic Route) Side Effects". Mayo Clinic. Archived from the original on 25 February 2023. Retrieved 16 March 2022.
- ^ "Common and Rare Side Effects for prednisolone acetate ophthalmic (eye)". www.webmd.com. Archived from the original on 21 May 2022. Retrieved 16 March 2022.
- ^ a b c "Pred Forte- prednisolone acetate suspension/ drops". DailyMed. 19 July 2019. Archived from the original on 9 March 2020. Retrieved 9 March 2020.
- ^ a b "Pred Mild- prednisolone acetate suspension/ drops". DailyMed. 19 July 2019. Archived from the original on 9 March 2020. Retrieved 9 March 2020.
- ^ Herretes S, Wang X, Reyes JM (October 2014). "Topical corticosteroids as adjunctive therapy for bacterial keratitis". The Cochrane Database of Systematic Reviews. 2014 (10): CD005430. doi:10.1002/14651858.CD005430.pub3. PMC 4269217. PMID 25321340.
- ^ Arslan N, Tepe D, Taştan E, Demirci M, Caydere M, Ustun H, Oguz H (November 2012). "Evaluation of the effectiveness of topical ciprofloxacin and prednisolone in the prevention of myringosclerosis". European Archives of Oto-Rhino-Laryngology. 269 (11): 2335–2341. doi:10.1007/s00405-011-1889-z. PMID 22197890. S2CID 23472925.
- ^ Wilhelmus KR, Gee L, Hauck WW, Kurinij N, Dawson CR, Jones DB, et al. (April 2020). "Herpetic Eye Disease Study: A Controlled Trial of Topical Corticosteroids for Herpes Simplex Stromal Keratitis". Ophthalmology. 127 (4S): S5–S18. doi:10.1016/j.ophtha.2020.01.037. PMID 32200827. S2CID 214616647.
- ^ Gayam K, Ramulu PY, Rengaraj V, Srinivasan K (29 February 2020). "Safety and Efficacy of 0.1% Nepafenac versus 1% Prednisolone Acetate Eye Drops after Laser Peripheral Iridotomy: A Prospective, Randomized Trial". Ophthalmology. Glaucoma. 3 (3): 174–180. doi:10.1016/j.ogla.2020.02.006. PMID 32672612. S2CID 90234022.
- ^ a b "BNF: Prednisolone, Medicine Forms". Medicines Complete. Archived from the original on 16 March 2022. Retrieved 14 April 2022.
- ^ a b "Flo-Pred- prednisolone acetate suspension". DailyMed. 28 February 2019. Archived from the original on 9 May 2021. Retrieved 9 March 2020.
- ^ a b c d "Pediapred- prednisolone sodium phosphate solution". DailyMed. 29 January 2020. Archived from the original on 9 May 2021. Retrieved 9 March 2020.
- ^ "Prednisolone (Oral Route)". Mayo Clinic. Retrieved 1 August 2023.
- ^ ReMine SG, McIlrath DC (1980). "Bowel perforation in steroid-treated patients". Annals of Surgery. 192 (4): 581–6. doi:10.1097/00000658-198010000-00016. PMC 1347010. PMID 7425698.
- ^ Cervin A, Andersson M (September 1998). "Intranasal steroids and septum perforation--an overlooked complication? A description of the course of events and a discussion of the causes". Rhinology. 36 (3): 128–32. PMID 9830677.
- ^ Goldstein BG, Goldstein AO (13 September 2022). Dellavalle RP, Levy ML, Corona R (eds.). "Topical corticosteroids: Use and adverse effects".
- ^ Stahn C, Löwenberg M, Hommes DW, Buttgereit F (September 2007). "Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists" (PDF). Molecular and Cellular Endocrinology. 275 (1–2): 71–78. doi:10.1016/j.mce.2007.05.019. PMID 17630118. S2CID 4121936. Archived (PDF) from the original on 28 September 2020. Retrieved 27 June 2019.
- ^ a b Schijvens AM, Ter Heine R, de Wildt SN, Schreuder MF (March 2019). "Pharmacology and pharmacogenetics of prednisone and prednisolone in patients with nephrotic syndrome". Pediatric Nephrology. 34 (3): 389–403. doi:10.1007/s00467-018-3929-z. PMC 6349812. PMID 29549463.
- ^ "Prednisolone acetate". go.drugbank.com. Archived from the original on 25 February 2023. Retrieved 16 March 2022.
- ^ "What's the Difference Between Prednisolone and Prednisone?". Archived from the original on 10 October 2022. Retrieved 27 November 2022.
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 1011–1012. ISBN 978-1-4757-2085-3. Archived from the original on 10 September 2017.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 869–. ISBN 978-3-88763-075-1. Archived from the original on 10 September 2017.
- ^ "Prednisolone (Ophthalmic Route)". Mayo Foundation for Medical Education and Research.
- ^ "Prednisolone Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing - WebMD". www.webmd.com. Retrieved 29 February 2024.
- ^ a b c d e "Prednisolone ophthalmic Use During Pregnancy". Drugs.com. Archived from the original on 25 February 2023. Retrieved 16 March 2022.
- ^ "Prednisolone: Uses, Dosage, Side Effects, Warnings". Drugs.com. 6 July 2022.
- ^ "Prohibited List" (PDF). World Anti-Doping Agency. January 2020. Archived (PDF) from the original on 9 March 2020.
- ^ a b c d "PrednisoLONE Acetate Ophthalmic Suspension". www.vetrxdirect.com. Archived from the original on 25 February 2023. Retrieved 16 March 2022.
- ^ a b c "Prednisolone Acetate Ophthalmic for Veterinary Use". www.wedgewoodpharmacy.com. Archived from the original on 4 July 2022. Retrieved 16 March 2022.
- ^ Gollakner R. "Prednisolone/Prednisone". VCA Hospitals. Archived from the original on 21 June 2021. Retrieved 3 July 2021.
- ^ Videla R, Sommardahl C, Smith J, Schaefer DM, Cox S (2021). "Pharmacokinetics of Orally Administered Prednisolone in Alpacas". Frontiers in Veterinary Science. 8: 745890. doi:10.3389/fvets.2021.745890. PMC 8569471. PMID 34746285.
External links
edit- "Prednisolone Ophthalmic". MedlinePlus.
- US patent 2837464, Arthur Nobile, "Process for production of dienes by corynebacteria", published 1958-06-03, issued 1958-06-03, assigned to Schering Corp