Gadoteridol (INN) is a gadolinium-based MRI contrast agent, used particularly in the imaging of the central nervous system. It is sold under the brand name ProHance.[3] Gadoteridol was first approved for use in the United States in 1992.[4]
 | |
| Clinical data | |
|---|---|
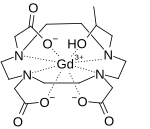
| Other names | (10-(2-(hydroxy-κO)propyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triacetato(3−)-κN1,κN4,κN7,κN10,κO1,κO4,κO7)-gadolinium |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | IV |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H29GdN4O7 |
| Molar mass | 558.69 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
References
edit- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ "Prohance Product information". Health Canada. 30 July 1998. Retrieved 19 April 2024.
- ^ Bracco Diagnostic Inc. (26 October 2022). "Gadoteridol (ProHance) prescribing information". DailyMed. U.S. National Library of Medicine. Retrieved 17 May 2023.
- ^ Morgan DE, Spann JS, Lockhart ME, Winningham B, Bolus DN (April 2011). "Assessment of adverse reaction rates during gadoteridol-enhanced MR imaging in 28,078 patients". Radiology. 259 (1): 109–16. doi:10.1148/radiol.10100906. PMID 21248237.
Specifically, the rate of nausea (0.530%) was less than half the rate (1.4%) in clinical trials of 1251 patients, leading to FDA approval in 1992.