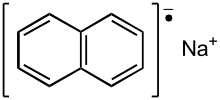
Sodium naphthalene is an organic salt with the chemical formula Na+[C10H8]−. In the research laboratory, it is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry. It is usually generated in situ. When isolated, it invariably crystallizes as a solvate with ligands bound to Na+.[1]
| |
| Names | |
|---|---|
| Preferred IUPAC name
Sodium naphthalenide | |
| Systematic IUPAC name
Sodium naphthalen-1-ide | |
| Other names
sodium naphthalenide, sodium naphthalide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.020.420 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Na+[C10H8]− | |
| Molar mass | 151.164 g·mol−1 |
| Appearance | Deep green crystals |
| Related compounds | |
Other anions
|
Lithium naphthalene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation and properties
editThe alkali metal naphthalene salts are prepared by stirring the metal with naphthalene in an ethereal solvent, usually as tetrahydrofuran or dimethoxyethane. The resulting salt is dark green.[2][3][4] The anion is a radical, giving a strong EPR signal near g = 2.0. Its deep green color arises from absorptions centered at 463 and 735 nm.
Several solvates of sodium naphthalenide have been characterized by X-ray crystallography. The effects are subtle, the outer pair of CH−CH bonds contract by 3 pm and the other nine C−C bonds elongate by 2–3 pm. The net effect is that reduction weakens the bonding.[5][6]
Reactions
editRedox
editWith a reduction potential near −2.5 V vs NHE, the naphthalene radical anion is a strong reducing agent.[1] It is capable of defluorinating PTFE and is commonly used for chemically etching PTFE to allow adhesion.
Protonation
editThe anion is strongly basic, and a typical degradation pathway involves reaction with water and related protic sources such as alcohols. These reactions afford dihydronaphthalene:
As a ligand
editAlkali metal salts of the naphthalene radical anion are used to prepare complexes of naphthalene.[7]
Related reagents
editReferences
edit- ^ a b Connelly, Neil G.; Geiger, William E. (1996). "Chemical Redox Agents for Organometallic Chemistry". Chemical Reviews. 96 (2): 877–910. doi:10.1021/cr940053x. PMID 11848774.
- ^ Corey, E. J.; Gross, Andrew W. (1987). "tert-Butyl-tert-octylamine". Org. Syntheses. 65: 166. doi:10.15227/orgsyn.065.0166.
- ^ Cotton, F. Albert; Wilkinson, Geoffrey (1988), Advanced Inorganic Chemistry (5th ed.), New York: Wiley-Interscience, p. 139, ISBN 0-471-84997-9
- ^ Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 111. ISBN 978-0-08-022057-4.
- ^ Bock, Hans; Arad, Claudia; Näther, Christian; Havlas, Zdenek (1995). "The Structures of Solvent-Separated Naphthalene and Anthracene Radical Anions". J. Chem. Soc., Chem. Commun. (23): 2393–2394. doi:10.1039/C39950002393.
- ^ Castillo, Maximiliano; Metta-Magaña, Alejandro J.; Fortier, Skye (2016). "Isolation of Gravimetrically Quantifiable Alkali Metal Arenides Using 18-Crown-6". New Journal of Chemistry. 40 (3): 1923–1926. doi:10.1039/C5NJ02841H.
- ^ Ellis, John E. (2019). "The Chatt Reaction: Conventional Routes to homoleptic Arenemetalates of d-Block Elements". Dalton Transactions. 48 (26): 9538–9563. doi:10.1039/C8DT05029E. PMID 30724934. S2CID 73436073.