Thallium(I) sulfate (Tl2SO4) or thallous sulfate is the sulfate salt of thallium in the common +1 oxidation state, as indicated by the Roman numeral I. It is often referred to as simply thallium sulfate.[2]
| |
| Names | |
|---|---|
| Other names
Thallous sulfate, Thallium sulfate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.365 |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1707 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Tl2SO4 | |
| Molar mass | 504.83 g/mol |
| Appearance | white prisms or dense white powder |
| Odor | odorless |
| Density | 6.77 g/cm3 |
| Melting point | 632 °C (1,170 °F; 905 K) |
| 2.70 g/100 mL (0 °C) 4.87 g/100 mL (20 °C) 18.45 g/100 mL (100 °C) | |
| −112.6·10−6 cm3/mol | |
Refractive index (nD)
|
1.860 |
| Structure | |
| rhomboid | |
| Hazards | |
| GHS labelling: | |
   
| |
| Danger | |
| H300, H315, H372, H411 | |
| P260, P264, P270, P273, P280, P301+P310, P302+P352, P314, P321, P330, P332+P313, P362, P391, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
16 mg/kg (rat, oral) 23.5 mg/kg (mouse, oral)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Uses
editDuring the last two centuries, Tl2SO4 had been used for various medical treatments but was abandoned. In the later 1900s it found use mainly for rodenticides.[3] These applications were prohibited in 1975 in the US due to the nonselective nature of its toxicity. Thallium(I) sulfate inhibits the growth of plants by preventing germination. Tl2SO4 is mostly used today as a source of Tl+ in the research laboratory. It is a precursor to thallium(I) sulfide (Tl2S), which exhibits high electrical conductivity when exposed to infrared light.
Preparation
editThallium(I) sulfate is produced by the reaction of thallium metal with sulfuric acid followed by crystallization.
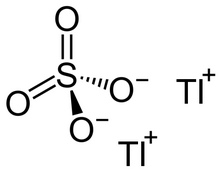
Structure
editTl2SO4 adopts the same structure as K2SO4. In aqueous solution, the thallium(I) cations and the sulfate anions are separated and highly solvated. Thallium(I) sulfate crystals have a C2 symmetry.
Toxicity
editThallium(I) sulfate is soluble in water and its toxic effects are derived from the thallium(I) cation. The mean lethal dose of thallium(I) sulfate for an adult is about 1 gram. Since thallium(I) sulfate is a simple powder with indistinctive properties, it can easily be mistaken for more innocuous chemicals. It can enter the body by ingestion, inhalation, or through contact with the skin. The thallium(I) cation is very similar to potassium and sodium cations, which are essential for life. After the thallium ion enters the cell, many of the processes that transport potassium and sodium are disrupted. Due to its poisonous nature, many western countries have banned the use of thallium(I) sulfate in products for home use and many companies have also stopped using this compound.
A dosage in excess of 500 mg is reported as fatal. Thallium(I) sulfate, after entering the body, concentrates itself in the kidneys, liver, brain, and other tissues in the body.
Thallium(I) sulfate was used in Israel to control the rodent population; it is suspected that in the 1950s, this resulted in the disappearance of the brown fish owl.[4]
Sources
editReferences
edit- ^ "Thallium (soluble compounds, as Tl)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Micke, Heinrich; Wolf, Hans Uwe (2000). "Thallium and Thallium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_607. ISBN 978-3527306732.
- ^ "World Health Organization Pesticide Data Sheet no.10 (1975)". Archived from the original on 2008-02-24. Retrieved 2006-02-28.
- ^ Mendelssohn, H. Ecological effects of chemical control of rodents and jackals in Israel Archived 2014-02-02 at the Wayback Machine at LPO Mission Rapaces
External links
edit- International Chemical Safety Card 0336
- NIOSH Pocket Guide to Chemical Hazards
- Pesticide Data Sheet Archived 2008-02-24 at the Wayback Machine (WHO/FAO)
- Kaunas University of Technology
- University of Wisconsin-Madison Chemistry Department
- Smithsonian National Zoological Park Archived 2008-03-03 at the Wayback Machine
- ISIS Conducting Chemical Weapons Tests on Live Victims
