Aconitic acid is an organic acid. The two isomers are cis-aconitic acid and trans-aconitic acid. The conjugate base of cis-aconitic acid, cis-aconitate is an intermediate in the isomerization of citrate to isocitrate in the citric acid cycle. It is acted upon by the enzyme aconitase.
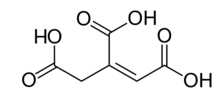 cis-aconitic acid
| |
 trans-aconitic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
Prop-1-ene-1,2,3-tricarboxylic acid | |
| Other names
Achilleic acid; equisetic acid; citridinic acid; pyrocitric acid; achilleaic acid; acinitic acid
| |
| Identifiers | |
| ChemSpider |
|
| ECHA InfoCard | 100.007.162 |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C6H6O6 | |
| Molar mass | 174.108 g·mol−1 |
| Appearance | Colorless crystals |
| Melting point | 190 °C (374 °F; 463 K) (decomposes) (mixed isomers), 173 °C (cis and trans isomers) |
| Acidity (pKa) | 2.80, 4.46 (trans isomer)[2] 2.78, 4.41, 6.21 (cis isomer)[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Aconitic acid can be synthesized by dehydration of citric acid using sulfuric acid:[4]
- (HO2CCH2)2C(OH)CO2H → HO2CCH=C(CO2H)CH2CO2H + H2O
A mixture of isomers are generated in this way.
Aconitic acid was originally isolated from Aconitum napellus by Swiss chemist and apothecary Jacques Peschier in 1820.[5][6] It was first prepared by thermal dehydration.[7]
Like the conjugate bases of other polycarboxylic acid, acotinic acid forms a variety of coordination complexes. One example is the coordination polymer [Zn3(C6H3O6)2(H2O)6]n.[8]
References
edit- ^ "Aconitic Acid - Compound Summary (CID 309)". PubChem.
- ^ Dawson, R. M. C.; Elliott, D. C.; Elliott, W. H. (1989). Data for Biochemical Research (3rd ed.). Oxford: Clarendon Press. ISBN 9780198552994.
- ^ Pfendt, L.; Dražić, B.; Popović, G.; Drakulić, B.; Vitnik, Ž; Juranić, I. (2003). "Determination of all pKa values of some di- and tri-carboxylic unsaturated and epoxy acids and their polylinear correlation with the carboxylic group atomic charges". Journal of Chemical Research. 2003: 247–248. doi:10.3184/030823403103173732.
- ^ Bruce, W. F. (1937). "Aconitic Acid". Organic Syntheses. 17: 1. doi:10.15227/orgsyn.017.0001.
- ^ Brande, William Thomas (1848). A Manual of Chemistry, Vol II (6 ed.). London: John W. Parker. p. 1344. Retrieved 8 November 2023.
- ^ Reichenbach, Karl-Rudolf (2001). Jacques Peschier (1769-1832): Ein Genfer Apotheker und Chemiker. Zürich: Wissenschaftliche Verlagsgesellschaft mbH Stuttgart. ISBN 3804719090. Retrieved 8 November 2023.
- ^ Pawolleck, B. (1875). "Substitutionsproducte der Citronensäure und ein Versuch zur Synthese der letzteren" [Substitution products of citric acid and an attempt at the synthesis of the latter]. Justus Liebig's Annalen der Chemie. 178 (2–3): 150–170. doi:10.1002/jlac.18751780203.
- ^ Zhang, Kou-Lin; Zhou, Fang; Yuan, Li-Min; Diao, Guo-Wang; Ng, Seik Weng (2009). "Synthesis and characterization of a novel photoluminescent three-dimensional metal–organic framework". Inorganica Chimica Acta. 362 (7): 2510–2514. doi:10.1016/j.ica.2008.10.008.