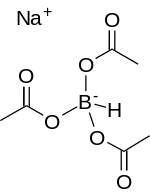
Sodium triacetoxyborohydride, also known as sodium triacetoxyhydroborate, commonly abbreviated STAB, is a chemical compound with the formula Na[(CH3COO)3BH]. Like other borohydrides, it is used as a reducing agent in organic synthesis. This colourless salt is prepared by protonolysis of sodium borohydride with acetic acid:[1]
| |
| Names | |
|---|---|
| Other names
NaBH(OAc)3; STAB; STABH; Sodium triacetoxyhydroborate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.115.747 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Na[(CH3COO)3BH] | |
| Molar mass | 211.94 g·mol−1 |
| Appearance | White powder |
| Density | 1.20 g/cm3 |
| Melting point | 116 to 120 °C (241 to 248 °F; 389 to 393 K) decomposes |
| decomposition | |
| Structure | |
| 4 at boron atom | |
| Tetrahedral at boron atom | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Other anions
|
Sodium cyanoborohydride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
- Na[BH4] + 3 CH3COOH → Na[(CH3COO)3BH] + 3 H2
Comparison with related reagents
editSodium triacetoxyborohydride is a milder reducing agent than sodium borohydride or even sodium cyanoborohydride. It reduces aldehydes but not most ketones. It is especially suitable for reductive aminations of aldehydes and ketones.[2][3][4]
However, unlike sodium cyanoborohydride, the triacetoxyborohydride hydrolyzes readily, nor is it compatible with methanol. It reacts only slowly with ethanol and isopropanol and can be used with these.[3]
NaBH(OAc)3 may also be used for reductive alkylation of secondary amines with aldehyde-bisulfite adducts. [5]
Monoacetoxyborohydride
editThe combination of Na[BH4] with carboxylic acids results in the formation of acyloxyborohydride species other than sodium triacetoxyborohydride. These modified species can perform a variety of reductions not normally associated with borohydride chemistry, such as alcohols to hydrocarbons and nitriles to primary amines.[6]
See also
edit- Sodium cyanoborohydride - a slightly stronger reductant, but amenable to protic solvents
- Sodium borohydride - a stronger, cheaper reductant
- Tetramethylammonium triacetoxyborohydride[7]
References
edit- ^ Gordon W. Gribble, Ahmed F. Abdel-Magid, "Sodium Triacetoxyborohydride" Encyclopedia of Reagents for Organic Synthesis, 2007, John Wiley & Sons.doi:10.1002/047084289X.rs112.pub2
- ^ Abdel-Magid, A. F.; Carson, K. G.; Harris, B. D.; Maryanoff, C. A.; Shah, R. D. (1996). "Reductive Amination of Aldehydes and Ketones with Sodium Triacetoxyborohydride. Studies on Direct and Indirect Reductive Amination Procedures1". The Journal of Organic Chemistry. 61 (11): 3849–3862. doi:10.1021/jo960057x. PMID 11667239.
- ^ a b Abdel-Magid, A. F.; Mehrman, S. J. (2006). "A Review on the Use of Sodium Triacetoxyborohydride in the Reductive Amination of Ketones and Aldehydes". Organic Process Research & Development. 10 (5): 971. doi:10.1021/op0601013.
- ^ Magano, Javier; Kiser, E. Jason; Shine, Russell J.; Chen, Michael H. (2013). "Oxindole Synthesis via Palladium-catalyzed C-H Functionalization". Organic Syntheses. 90: 74. doi:10.15227/orgsyn.090.0074.
- ^ Pandit, C. R.; Mani, N. S. (2009). "Expedient reductive amination of aldehyde bisulfite adducts". Synthesis (23): 4032–4036.
- ^ Gribble, Gordon, W. (1998). "Sodium borohydride in carboxylic acid media: a phenomenal reduction system". Chemical Society Reviews. 27 (6): 395. doi:10.1039/A827395Z. S2CID 96906861.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Houri, Ahmad F.; Hoveyda, Amir H. (2001). "Tetramethylammonium Triacetoxyborohydride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt059. ISBN 0-471-93623-5.
