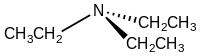
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA is also a common abbreviation.[8][9] It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Like diisopropylethylamine (Hünig's base), triethylamine is commonly employed in organic synthesis, usually as a base.
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
N,N-Diethylethanamine | |||
| Other names
(Triethyl)amine
Triethylamine (no longer IUPAC name[1]) | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | TEA[2] | ||
| 605283 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.064 | ||
| EC Number |
| ||
| KEGG | |||
| MeSH | triethylamine | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1296 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties[5] | |||
| C6H15N | |||
| Molar mass | 101.193 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Fishy, ammoniacal | ||
| Density | 0.7255 g mL−1 | ||
| Melting point | −114.70 °C; −174.46 °F; 158.45 K | ||
| Boiling point | 88.6 to 89.8 °C; 191.4 to 193.5 °F; 361.7 to 362.9 K | ||
| 112.4 g/L at 20 °C[3] | |||
| Solubility | miscible with organic solvents | ||
| log P | 1.647 | ||
| Vapor pressure | 6.899–8.506 kPa | ||
Henry's law
constant (kH) |
66 μmol Pa−1 kg−1 | ||
| Acidity (pKa) | 10.75 (for the conjugate acid) (H2O), 9.00 (DMSO)[4] | ||
| -81.4·10−6 cm3/mol | |||
Refractive index (nD)
|
1.401 | ||
| Thermochemistry | |||
Heat capacity (C)
|
216.43 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−169 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−4.37763 to −4.37655 MJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H225, H302, H312, H314, H332 | |||
| P210, P280, P305+P351+P338, P310 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −15 °C (5 °F; 258 K) | ||
| 312 °C (594 °F; 585 K) | |||
| Explosive limits | 1.2–8% | ||
Threshold limit value (TLV)
|
2 ppm (8 mg/m3) (TWA), 4 ppm (17 mg/m3) (STEL) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
| ||
LCLo (lowest published)
|
1425 ppm (mouse, 2 hr)[7] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 25 ppm (100 mg/m3)[6] | ||
REL (Recommended)
|
None established[6] | ||
IDLH (Immediate danger)
|
200 ppm[6] | ||
| Related compounds | |||
Related amines
|
|||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Synthesis and properties
editTriethylamine is prepared by the alkylation of ammonia with ethanol:[10]
- NH3 + 3 C2H5OH → N(C2H5)3 + 3 H2O
The pKa of protonated triethylamine is 10.75,[4] and it can be used to prepare buffer solutions at that pH. The hydrochloride salt, triethylamine hydrochloride (triethylammonium chloride), is a colorless, odorless, and hygroscopic powder, which decomposes when heated to 261 °C.
Triethylamine is soluble in water to the extent of 112.4 g/L at 20 °C.[3] It is also miscible in common organic solvents, such as acetone, ethanol, and diethyl ether.
Laboratory samples of triethylamine can be purified by distilling from calcium hydride.[11]
In alkane solvents triethylamine is a Lewis base that forms adducts with a variety of Lewis acids, such as I2 and phenols. Owing to its steric bulk, it forms complexes with transition metals reluctantly.[12][13][14]
Applications
editTriethylamine is commonly employed in organic synthesis as a base. For example, it is commonly used as a base during the preparation of esters and amides from acyl chlorides.[15] Such reactions lead to the production of hydrogen chloride which combines with triethylamine to form the salt triethylamine hydrochloride, commonly called triethylammonium chloride. (R, R' = alkyl, aryl):
- R2NH + R'C(O)Cl + Et3N → R'C(O)NR2 + Et3NH+Cl−
Like other tertiary amines, it catalyzes the formation of urethane foams and epoxy resins. It is also useful in dehydrohalogenation reactions and Swern oxidations.
Triethylamine is readily alkylated to give the corresponding quaternary ammonium salt:
- RI + Et3N → Et3NR+I−
Triethylamine is mainly used in the production of quaternary ammonium compounds for textile auxiliaries and quaternary ammonium salts of dyes. It is also a catalyst and acid neutralizer for condensation reactions and is useful as an intermediate for manufacturing medicines, pesticides and other chemicals.
Triethylamine salts, like any other tertiary ammonium salts, are used as an ion-interaction reagent in ion interaction chromatography, due to their amphiphilic properties. Unlike quaternary ammonium salts, tertiary ammonium salts are much more volatile, therefore mass spectrometry can be used while performing analysis.
Niche uses
editTriethylamine is commonly used in the production of anionic Polyurethane dispersions (resins dispersed in water rather than solvents) as a neutralizing agent.
Triethylamine is used to give salts of various carboxylic acid-containing pesticides, e.g. Triclopyr and 2,4-dichlorophenoxyacetic acid.[citation needed]
Triethylamine is the active ingredient in FlyNap, a product for anesthetizing fruit flies.[citation needed] It is also used in mosquito and vector control labs to anesthetize mosquitoes. This is done to preserve any viral material that might be present during species identification.[citation needed][how?]
The bicarbonate salt of triethylamine (often abbreviated TEAB, triethylammonium bicarbonate) is useful in reverse phase chromatography, often in a gradient to purify nucleotides and other biomolecules.[citation needed]
Triethylamine was discovered by the Germans during the early 1940s to be hypergolic in combination with nitric acid, and was used as a component in the German Wasserfall rocket.[16] The Soviet Scud missile used TONKA-250, a mixture of 50% xylidine and 50% triethylamine as a starting fluid to ignite its rocket engine.[17][better source needed]
Natural occurrence
editHawthorn flowers have a heavy, complicated scent, the distinctive part of which is triethylamine, which is also one of the first chemicals produced by a dead human body when it begins to decay. Due to the scent, it is considered unlucky in British culture to bring hawthorn into a house. Gangrene and semen are also said to possess a similar odour.[18]
References
edit- ^ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 671. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ X. Bories-Azeau, S. P. Armes, and H. J. W. van den Haak, Macromolecules 2004, 37, 2348 PDF
- ^ a b "MSDS - 471283". www.sigmaaldrich.com. Retrieved 2020-06-17.
- ^ a b David Evans Research Group Archived 2012-01-21 at the Wayback Machine
- ^ The Merck Index (11th ed.). 9582.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0633". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Triethylamine". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "Ethanolamine Compounds (MEA, DEA, TEA And Others)". Safe Cosmetics. Retrieved 2020-06-17.
- ^ "tetraethylammonium | Ligand page | IUPHAR/BPS Guide to PHARMACOLOGY". www.guidetopharmacology.org. Retrieved 2020-06-17.
- ^ Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2000). Amines, Aliphatic. doi:10.1002/14356007.a02_001. ISBN 3-527-30673-0.
- ^ F., Armarego, W. L. (2012-10-17). Purification of Laboratory Chemicals. Chai, Christina Li Lin (Seventh ed.). Amsterdam. ISBN 978-0-12-382162-1. OCLC 820853648.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: multiple names: authors list (link) - ^ DeLaive, Patricia J.; Sullivan, B. P.; Meyer, T. J.; Whitten, D. G. (July 1979). "Applications of light-induced electron-transfer reactions. Coupling of hydrogen generation with photoreduction of ruthenium(II) complexes by triethylamine". Journal of the American Chemical Society. 101 (14): 4007–4008. doi:10.1021/ja00508a070. ISSN 0002-7863.
- ^ DeLaive, Patricia J.; Foreman, Thomas K.; Giannotti, Charles; Whitten, David G. (August 1980). "Photoinduced electron transfer reactions of transition-metal complexes with amines. Mechanistic studies of alternate pathways to back electron transfer". Journal of the American Chemical Society. 102 (17): 5627–5631. doi:10.1021/ja00537a037. ISSN 0002-7863.
- ^ Seligson, Allen L.; Trogler, William C. (March 1991). "Cone angles for amine ligands. X-ray crystal structures and equilibrium measurements for ammonia, ethylamine, diethylamine, and triethylamine complexes with the [bis(dimethylphosphino)ethane]methylpalladium(II) cation". Journal of the American Chemical Society. 113 (7): 2520–2527. doi:10.1021/ja00007a028. ISSN 0002-7863.
- ^ Sorgi, K. L. (2001). "Triethylamine". Encyclopedia of Reagents for Organic Synthesis. New York: John Wiley & Sons. doi:10.1002/047084289X.rt217. ISBN 978-0-471-93623-7.
- ^ Clark, John Drury (23 May 2018). Ignition!: An Informal History of Liquid Rocket Propellants. Rutgers University Press. p. 302. ISBN 978-0-8135-9918-2.
- ^ Brügge, Norbert (24 February 2020). "The Soviet "Scud" missile family". b14643.de. Retrieved 29 July 2022.
- ^ The book of general ignorance. John Lloyd & John Mitchinson. Faber & Faber 2006, The Hawthorn, BBC


