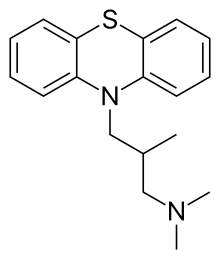
Alimemazine (INN), also known as trimeprazine, commonly provided as a tartrate salt, is a phenothiazine derivative that is used as an antipruritic (it prevents itching from causes such as eczema or poison ivy, by acting as an antihistamine).[3] It also acts as a sedative, hypnotic, and antiemetic for prevention of motion sickness. Although it is structurally related to drugs such as chlorpromazine, it is not used as an antipsychotic.[4] In the Russian Federation, it is marketed under the brand name Teraligen for the treatment of anxiety disorders (including GAD), organic mood disorders, sleep disturbances, personality disorders accompanied by asthenia and depression, somatoform autonomic dysfunction and various neuroses.[5]
 | |
 | |
| Clinical data | |
|---|---|
| Other names | trimeprazine, trimeprazine (BAN UK), trimeprazine (USAN US) |
| AHFS/Drugs.com | International Drug Names |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Anti-allergic agent |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Liver |
| Elimination half-life | 4.78 ± 0.59 hours[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.434 |
| Chemical and physical data | |
| Formula | C18H22N2S |
| Molar mass | 298.45 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Alimemazine is not approved for use in humans in the United States. The combination of alimemazine and prednisolone (commonly sold under the brand name Temaril-P) is licensed as an antipruritic and antitussive in dogs.[6] A generic version of the combination trimeprazine/prednisolone was approved by the US Food and Drug Administration (FDA) in June 2024.[7][8]
Society and culture
editBrand names
editBrand names include Nedeltran, Panectyl, Repeltin, Teraligen, Therafene, Theraligene, Theralen, Thegalin, Theralene, Vallergan, Vanectyl, and Temaril.
References
edit- ^ "Trimeprazine tartrate powder". DailyMed. 14 March 2019. Retrieved 15 July 2024.
- ^ Hu OY, Gfeller E, Perrin JH, Curry SH (March 1986). "Relative bioavailability of trimeprazine tablets investigated in man using HPLC with electrochemical detection". The Journal of Pharmacy and Pharmacology. 38 (3): 172–6. doi:10.1111/j.2042-7158.1986.tb04539.x. PMID 2871150. S2CID 506087.
- ^ "(3R,4R)-3,4-dihydroxyhexane-2,5-dione;N,N,2-trimethyl-3-phenothiazin-10-ylpropan-1-amine". pubchem.ncbi.nlm.nih.gov.
- ^ "Drugbank:Trimeprazine". Archived from the original on 2006-12-30. Retrieved 2007-09-15.
- ^ "Тералиджен — инструкция по применению, дозы, побочные действия, описание препарата: таблетки, покрытые пленочной оболочкой,раствор для внутримышечного введения, 5 мг/мл, 5 мг". РЛС®.
- ^ "Temaril-P- trimeprazine tartrate and prednisolone tablet". DailyMed. 12 January 2023. Retrieved 15 July 2024.
- ^ "FDA Roundup: June 21, 2024". U.S. Food and Drug Administration (FDA). 21 June 2024. Retrieved 15 July 2024.
- ^ "FOI Summary for the Original Approval of ANADA 200-784". United States Food and Drug Administration. Archived from the original on 15 July 2024. Retrieved 29 September 2024.