Voltage-dependent anion channels, or mitochondrial porins, are a class of porin ion channel located on the outer mitochondrial membrane.[1][2] There is debate as to whether or not this channel is expressed in the cell surface membrane.[3][4][5]
| Eukaryotic porin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
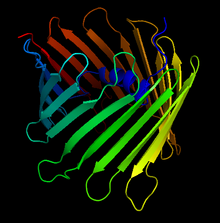 Crystal Structure of the Human Voltage-Dependent Anion Channel. The arrows denote the antiparallel beta sheets that form the characteristic beta-barrel | |||||||||
| Identifiers | |||||||||
| Symbol | Porin_3 | ||||||||
| Pfam | PF01459 | ||||||||
| InterPro | IPR001925 | ||||||||
| PROSITE | PDOC00483 | ||||||||
| TCDB | 1.B.8 | ||||||||
| OPM superfamily | 189 | ||||||||
| OPM protein | 3emn | ||||||||
| CDD | cd07306 | ||||||||
| |||||||||
This major protein of the outer mitochondrial membrane of eukaryotes forms a voltage-dependent anion-selective channel (VDAC) that behaves as a general diffusion pore for small hydrophilic molecules.[6][7][8][9] The channel adopts an open conformation at low or zero membrane potential and a closed conformation at potentials above 30–40 mV. VDAC facilitates the exchange of ions and molecules between mitochondria and cytosol and is regulated by the interactions with other proteins and small molecules.[10]
Structure
editThis protein contains about 280 amino acids and forms a beta barrel which spans the mitochondrial outer membrane.[11][12]
Since its discovery in 1976, extensive function and structure analysis of VDAC proteins has been conducted. A prominent feature of the pore emerged: when reconstituted into planar lipid bilayers, there is a voltage-dependent switch between an anion-selective high-conductance state with high metabolite flux and a cation-selective low-conductance state with limited passage of metabolites.
More than 30 years after its initial discovery, in 2008, three independent structural projects of VDAC-1 were completed. The first was solved by multi-dimensional NMR spectroscopy. The second applied a hybrid approach using crystallographic data. The third was for mouse VDAC-1 crystals determined by X-ray crystallographic techniques. The three projects of the 3D structures of VDAC-1 revealed many structural features. First, VDAC-1 represents a new structural class of outer membrane β-barrel proteins with an odd number of strands. Another aspect is that the negatively charged side chain of residue E73 is oriented towards the hydrophobic membrane environment. The 19-stranded 3D structure obtained under different experimental sources by three different laboratories fits the EM and AFM data from native membrane sources and represents a biologically relevant state of VDAC-1.[10]
Mechanism
editAt membrane potentials exceeding 30 mV (positive or negative), VDAC assumes a closed state, and transitions to its open state once the voltage drops below this threshold. Although both states allow passage of simple salts, VDAC is much more stringent with organic anions, a category into which most metabolites fall.[13] The precise mechanism for coupling voltage changes to conformational changes within the protein has not yet been worked out, but studies by Thomas et al. suggest that when the protein transitions to the closed form, voltage changes lead to the removal of a large section of the protein from the channel and decrease effective pore radius.[14] Several lysine residues, as well as Glu-152, have been implicated as especially important sensor residues within the protein.[15]
Biological function
editThe voltage-dependent ion channel plays a key role in regulating metabolic and energetic flux across the outer mitochondrial membrane. It is involved in the transport of ATP, ADP, pyruvate, malate, and other metabolites, and thus communicates extensively with enzymes from metabolic pathways.[13] The ATP-dependent cytosolic enzymes hexokinase, glucokinase, and glycerol kinase, as well as the mitochondrial enzyme creatine kinase, have all been found to bind to VDAC. This binding puts them in close proximity to ATP released from the mitochondria. In particular, the binding of hexokinase is presumed to play a key role in coupling glycolysis to oxidative phosphorylation.[14] Additionally, VDAC is an important regulator of Ca2+ transport in and out of the mitochondria. Because Ca2+ is a cofactor for metabolic enzymes such as pyruvate dehydrogenase and isocitrate dehydrogenase, energetic production and homeostasis are both affected by VDAC's permeability to Ca2+.[16]
Disease relevance
editVDAC has also been shown to play a role in apoptosis.[17] During apoptosis, VDAC modifies the mitochondrial permeability transition pore to release of apoptogenic factors such as cytochrome c. However, VDAC are not essential components of the mitochondrial permeability transition pore. Although cytochrome c plays an essential role in oxidative phosphorylation within the mitochondrion. In the cytosol it activates proteolytic enzymes called caspases, which play a major role in cell death.[18] Although the mechanism for VDAC-facilitated cytochrome c release has not yet been fully elucidated, some research suggests that oligomerization between individual subunits may create a large flexible pore through which cytochrome c can pass.[19] A more important factor is that release of cytochrome c is also regulated by the Bcl-2 protein family: Bax interacts directly with VDAC to increase pore size and promote cytochrome c release, while anti-apoptotic Bcl-xL produces the exact opposite effect.[20] In fact, it has been shown that antibodies that inhibit VDAC also interfere with Bax-mediated cytochrome c release in both isolated mitochondria and whole cells.[21] This key role in apoptosis suggests VDAC as a potential target for chemotherapeutic drugs.
Examples
editYeast contains two members of this family (genes POR1 and POR2); vertebrates have at least three members (genes VDAC1, VDAC2 and VDAC3).[11]
Humans, like most higher eukaryotes, encode three different VDACs; VDAC1, VDAC2, and VDAC3. Together with TOMM40 and TOMM40L they represent a family of evolutionarily related β-barrels.[22]
Plants have the largest number of VDACs. Arabidopsis encode four different VDACs but this number can be larger in other species.[23]
References
edit- ^ Hoogenboom BW, Suda K, Engel A, Fotiadis D (2007). "The supramolecular assemblies of voltage-dependent anion channels in the native membrane". J. Mol. Biol. 370 (2): 246–55. doi:10.1016/j.jmb.2007.04.073. PMID 17524423.
- ^ Blachly-Dyson, E; Forte, M (September 2001). "VDAC channels". IUBMB Life. 52 (3–5): 113–8. doi:10.1080/15216540152845902. PMID 11798022. S2CID 38314888.
- ^ Sabirov RZ, Merzlyak PG (June 2012). "Plasmalemmal VDAC controversies and maxi-anion channel puzzle". Biochim. Biophys. Acta. 1818 (6): 1570–80. doi:10.1016/j.bbamem.2011.09.024. PMID 21986486.
- ^ De Pinto, V.; Messina, A.; Lane, D. J. R.; Lawen, A. (2010). "Voltage-dependent anion-selective channel (VDAC) in the plasma membrane". FEBS Letters. 584 (9): 1793–1799. Bibcode:2010FEBSL.584.1793D. doi:10.1016/j.febslet.2010.02.049. PMID 20184885. S2CID 3391282.
- ^ Niehage, C.; Steenblock, C.; Pursche, T.; Bornhäuser, M.; Corbeil, D.; Hoflack, B. (2011). Borlongan, Cesario V (ed.). "The Cell Surface Proteome of Human Mesenchymal Stromal Cells". PLOS ONE. 6 (5): e20399. Bibcode:2011PLoSO...620399N. doi:10.1371/journal.pone.0020399. PMC 3102717. PMID 21637820.
- ^ Benz R (1994). "Permeation of hydrophilic solutes through mitochondrial outer membranes: review on mitochondrial porins". Biochim. Biophys. Acta. 1197 (2): 167–196. doi:10.1016/0304-4157(94)90004-3. PMID 8031826.
- ^ Mannella CA (1992). "The 'ins' and 'outs' of mitochondrial membrane channels". Trends Biochem. Sci. 17 (8): 315–320. doi:10.1016/0968-0004(92)90444-E. PMID 1384178.
- ^ Dihanich M (1990). "The biogenesis and function of eukaryotic porins". Experientia. 46 (2): 146–153. doi:10.1007/BF02027310. PMID 1689252. S2CID 2199583.
- ^ Forte M, Guy HR, Mannella CA (1987). "Molecular genetics of the VDAC ion channel: structural model and sequence analysis". J. Bioenerg. Biomembr. 19 (4): 341–350. doi:10.1007/BF00768537. PMID 2442148. S2CID 10219032.
- ^ a b Hiller S, Abramson J, Mannella C, Wagner G, Zeth K (September 2010). "The 3D structures of VDAC represent a native conformation". Trends Biochem. Sci. 35 (9): 514–21. doi:10.1016/j.tibs.2010.03.005. PMC 2933295. PMID 20708406.
- ^ a b Sampson MJ, Lovell RS, Davison DB, Craigen WJ (1996). "A novel mouse mitochondrial voltage-dependent anion channel gene localizes to chromosome 8". Genomics. 36 (1): 192–196. doi:10.1006/geno.1996.0445. PMID 8812436.
- ^ Zeth K (2010). "Structure and evolution of mitochondrial outer membrane proteins of beta-barrel topology". Biochim. Biophys. Acta. 1797 (6–7): 1292–9. doi:10.1016/j.bbabio.2010.04.019. PMID 20450883.
- ^ a b Blachly-Dyson, E. & Forte, M. (2001). "VDAC Channels". IUBMB Life. 52 (3–5): 113–18. doi:10.1080/15216540152845902. PMID 11798022. S2CID 38314888.
- ^ a b Colombini M, Blachly-Dyson E, Forte M (1996). "VDAC, a Channel in the Outer Mitochondrial Membrane". Ion Channels. Vol. 4. pp. 169–202. doi:10.1007/978-1-4899-1775-1_5. ISBN 978-1-4899-1777-5. PMID 8744209.
- ^ Thomas L, Blachly-Dyson E, Colombini M, Forte M (June 1993). "Mapping of residues forming the voltage sensor of the voltage-dependent anion-selective channel". Proc. Natl. Acad. Sci. U.S.A. 90 (12): 5446–9. Bibcode:1993PNAS...90.5446T. doi:10.1073/pnas.90.12.5446. PMC 46737. PMID 7685903.
- ^ Shoshan-Barmatz V; Gincel D. (2003). "The voltage-dependent anion channel: characterization, modulation, and role in mitochondrial function in cell life and death". Cell Biochem. Biophys. 39 (3): 279–92. doi:10.1385/CBB:39:3:279. PMID 14716081. S2CID 33628015.
- ^ Lemasters JJ; Holmuhamedov E. (2006). "Voltage-dependent anion channel (VDAC) as mitochondrial governator--thinking outside the box". Biochim. Biophys. Acta. 1762 (2): 181–90. doi:10.1016/j.bbadis.2005.10.006. PMID 16307870.
- ^ Tsujimoto Y, Shimizu S (2002). "The voltage-dependent anion channel: an essential player in apoptosis". Biochimie. 84 (2–3): 187–93. doi:10.1016/S0300-9084(02)01370-6. PMID 12022949.
- ^ Zalk R; Israelson A; Garty ES; Azoulay-Zohar H; Shoshan-Barmatz V. (2005). "Oligomeric states of the voltage-dependent anion channel and cytochrome c release from mitochondria". Biochem. J. 386 (1): 73–83. doi:10.1042/BJ20041356. PMC 1134768. PMID 15456403.
- ^ Shimizu S; Narita M; Tsujimoto Y. (1999). "Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC". Nature. 399 (6735): 483–7. Bibcode:1999Natur.399..483S. doi:10.1038/20959. PMID 10365962. S2CID 4423304.
- ^ Shimizu S; Matsuoka Y; Shinohara Y; Yoneda Y; Tsujimoto Y. (2001). "Essential role of voltage-dependent anion channel in various forms of apoptosis in mammalian cells". J. Cell Biol. 152 (2): 237–50. doi:10.1083/jcb.152.2.237. PMC 2199613. PMID 11266442.
- ^ Bay DC, Hafez M, Young MJ, Court DA (June 2012). "Phylogenetic and coevolutionary analysis of the β-barrel protein family comprised of mitochondrial porin (VDAC) and Tom40". Biochim. Biophys. Acta. 1818 (6): 1502–19. doi:10.1016/j.bbamem.2011.11.027. PMID 22178864.
- ^ Homblé F, Krammer E, Prevost M (June 2012). "Plant VDAC: facts and speculations". Biochim. Biophys. Acta. 1818 (6): 1486–501. doi:10.1016/j.bbamem.2011.11.028. PMID 22155681.
External links
edit- Voltage-Dependent+Anion+Channels at the U.S. National Library of Medicine Medical Subject Headings (MeSH)