The mitral valve (/ˈmaɪtrəl/ MY-trəl), also known as the bicuspid valve or left atrioventricular valve, is one of the four heart valves. It has two cusps or flaps and lies between the left atrium and the left ventricle of the heart. The heart valves are all one-way valves allowing blood flow in just one direction. The mitral valve and the tricuspid valve are known as the atrioventricular valves because they lie between the atria and the ventricles.[1]
| Mitral valve | |
|---|---|
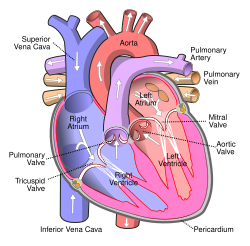 Anterior (frontal) view of the opened heart. White arrows indicate normal blood flow. (Mitral valve labeled at center right.) | |
 Base of ventricles exposed by removal of the atria. (Bicuspid (mitral) valve visible at bottom left.
Tricuspid valve visible at bottom right.) | |
| Details | |
| Identifiers | |
| Latin | valva atrioventricularis sinistra, valva mitralis, valvula bicuspidalis |
| MeSH | D008943 |
| TA98 | A12.1.04.003 |
| TA2 | 3987 |
| FMA | 7235 |
| Anatomical terminology | |
In normal conditions, blood flows through an open mitral valve during diastole with contraction of the left atrium, and the mitral valve closes during systole with contraction of the left ventricle. The valve opens and closes because of pressure differences, opening when there is greater pressure in the left atrium than ventricle and closing when there is greater pressure in the left ventricle than atrium.[2]
In abnormal conditions, blood may flow backward through the valve (mitral regurgitation) or the mitral valve may be narrowed (mitral stenosis). Rheumatic heart disease often affects the mitral valve; the valve may also prolapse with age and be affected by infective endocarditis. The mitral valve is named after the mitre of a bishop, which resembles its flaps.[3][4]
Structure
editThe mitral valve is typically 4 to 6 square centimetres (0.62 to 0.93 sq in) in area and sits in the left heart between the left atrium and the left ventricle.[5] It has two cusps: an anterior one, and a posterior one.[6] The opening of the mitral valve is surrounded by a fibrous ring known as the mitral annulus. The anterior cusp attaches to one third of the circumverence of the annulus, and the posterior cusp attaches to the remaining two thirds of its circumference. The anterior cusp is thicker and more rigid than the posterior one,[6] and covers approximately two-thirds of the valve.[citation needed] The anterior cusp intervenes between the mitral and aortic orifices.[6] Although the anterior leaflet takes up a larger part of the ring and rises higher, the posterior leaflet has a larger surface area.[citation needed]
Leaflets
editIn Carpentier's classification of a mitral valve, both the posterior and anterior mitral valve leaflets are divided into eight segments: P3 (medial scallop), P2 (middle scallop), P1 (lateral scallop), A3 (anteromedial segment), A2 (anteromedial), A1 (anterolateral), PMC (posteromedial commissure), ALC (anterolateral commissure).[7] Mitral leaflet thickness is usually about 1 mm but sometimes can range from 3–5 mm.[7][8]
Chordae tendineae
editThe valve leaflets are prevented from prolapsing into the left atrium by the action of chordae tendineae. The chordae tendineae are inelastic tendons attached at one end to papillary muscles in the left ventricle, and at the other to the valve cusps. Papillary muscles are finger-like projections from the wall of the left ventricle.
When the left ventricle contracts, the pressure in the ventricle forces the valve to close, while the tendons keep the leaflets coapting together and prevent the valve from opening in the wrong direction (thus preventing blood flowing back to the left atrium). Each chord has a different thickness. The thinnest ones are attached to the free leaflet margin, whereas the thickest ones (strut chords) are attached further from the free margin. This disposition has important effects on systolic stress distribution physiology.[9]
Annulus
editThe mitral annulus is a fibrous ring that is attached to the mitral valve leaflets. Unlike prosthetic valves, it is not continuous. The mitral annulus is saddle shaped and changes in shape throughout the cardiac cycle.[10] The annulus contracts and reduces its surface area during systole to help provide complete closure of the leaflets. Expansion of the annulus can result in leaflets that do not join soundly together, leading to functional mitral regurgitation.[11]
The normal diameter of the mitral annulus is 2.7 to 3.5 centimetres (1.1 to 1.4 in), and the circumference is 8 to 9 centimetres (3.1 to 3.5 in). Microscopically, there is no evidence of an annular structure anteriorly, where the mitral valve leaflet is contiguous with the posterior aortic root.[12]
Function
editDuring left ventricular diastole, after the pressure drops in the left ventricle due to relaxation of the ventricular myocardium, the mitral valve opens, and blood travels from the left atrium to the left ventricle. About 70 to 80% of the blood that travels across the mitral valve occurs during the early filling phase of the left ventricle. This early filling phase is due to active relaxation of the ventricular myocardium, causing a pressure gradient that allows a rapid flow of blood from the left atrium, across the mitral valve. This early filling across the mitral valve is seen on doppler echocardiography of the mitral valve as the E wave.
After the E wave, there is a period of slow filling of the ventricle.
Left atrial contraction (left atrial systole) (during left ventricular diastole) causes added blood to flow across the mitral valve immediately before left ventricular systole. This late flow across the open mitral valve is seen on doppler echocardiography of the mitral valve as the A wave. The late filling of the left ventricle contributes about 20% to the volume in the left ventricle prior to ventricular systole and is known as the atrial kick.
The mitral annulus changes in shape and size during the cardiac cycle. It is smaller at the end of atrial systole due to the contraction of the left atrium around it, like a sphincter. This reduction in annulus size at the end of atrial systole may be important for the proper coapting of the leaflets of the mitral valve when the left ventricle contracts and pumps blood.[13] Leaking valves can be corrected by mitral valve annuloplasty, a common surgical procedure that aims at restoring proper leaflet adjustment.
Clinical significance
editDisease
editThere are some valvular heart diseases that affect the mitral valve. Mitral stenosis is a narrowing of the valve. This can be heard as an opening snap; a heart sound which is not normally present.
Classic mitral valve prolapse is caused by an excess of connective tissue that thickens the spongiosa layer of the cusp and separates collagen bundles in the fibrosa. This weakens the cusps and adjacent tissue, resulting in an increased cuspal area and lengthening of the chordae tendineae. Elongation of the chordae tendineae often causes rupture, commonly to the chordae attached to the posterior cusp. Advanced lesions—also commonly involving the posterior leaflet—lead to leaflet folding, inversion, and displacement toward the left atrium.[14]
A valve prolapse can result in mitral insufficiency, which is the regurgitation or backflow of blood from the left ventricle to the left atrium due to the incomplete closure of the valve causing a systolic murmur heard at the apex of the heart. This increase in pressure in the left atrium and pulmonary circuit can lead to symptoms like fatigue, shortness of breath, and atrial fibrillation over time.[15]
Rheumatic heart disease often affects the mitral valve. The valve may also be affected by infective endocarditis.
There are also some rarer forms of congenital mitral valve disease that are often associated with other congenital heart anomalies. Parachute mitral valve occurs when all chordae tendineae of the mitral valve are abnormally attached to a single (or fused) papillary muscle. Straddling Mitral Valve occurs when the mitral valve's chordal attachments straddles, or goes through, a ventricular septal defect (VSD) and so has chordae originating on both sides of the ventricular septum. Mitral valve agenesis is very rare, defined as an absence or minimal presence of both mitral valve leaflets (complete agenesis) or one of the leaflets (partial agenesis).[16]
Surgery can be performed to replace or repair a damaged valve. A less invasive method is that of mitral valvuloplasty which uses a balloon catheter to open up a stenotic valve. Alternatively, the Lawrie technique is an option for patients who have less valve tissue available for repair as they may have damaged or fragile valve leaflets. During the Lawrie technique, artificial fabric chordae are used to repair the valve which spares the existing valve leaflets and chordae during the repair. [17]
Rarely there can be a severe form of calcification of the mitral valve annulus that can be mistaken for an intracardiac mass or thrombus.[18]
Mitral disease can be classified using Carpentier's classification which is based on the leaflet motion. Type I pertains to normal leaflet motion. Whereas, disease of the valve is categorized to primary mitral regurgitation or secondary mitral regurgitation based on the regurgitant etiology. Type II pertains to excessive leaflet motion leading to leaflet prolapse. Common causes include, but is not limited to, Barlow disease, myxomatous degeneration, inflammation, and papillary muscle rupture. Type III pertains to restrictive motion of the leaflets. Type IIIa pertains to restrictive motion during systole and diastole. Type IIIb pertains to restrictive motion during systole.[19]
Investigation
editThe closing of the mitral valve and the tricuspid valve constitutes the first heart sound (S1), which can be heard with a stethoscope. It is not the valve closure itself which produces the sound but the sudden cessation of blood flow, when the mitral and tricuspid valves close.[citation needed]. Abnormalities associated with the mitral valve can often be heard when listening with a stethoscope.
The mitral valve is often also investigated using an ultrasound scan, which can reveal the size, anatomy and flow of blood through the valve.
Etymology
editThe word mitral comes from Latin, meaning "shaped like a mitre" (bishop's hat). The word bicuspid uses combining forms of bi-, from Latin, meaning "double", and cusp, meaning "point", reflecting the dual-flap shape of the valve.
Additional images
edit-
The human heart, viewed from the front. The mitral valve is visible on the right as the "bicuspid valve"
-
Mitral valve, viewed in a cadaver specimen from within the left atrium.
See also
editReferences
edit- ^ Standring, Susan, ed. (2016). Gray's Anatomy: The Anatomical Basis of Clinical Practice. Philadelphia. ISBN 9780702052309. OCLC 920806541.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Guyton, Arthur C.; Hall, John E. (2011). Guyton and Hall's Textbook of medical physiology (Twelfth ed.). Philadelphia, Pa. ISBN 9781416045748. OCLC 434319356.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ NORRIS, TOMMIE L. (2018). PORTH'S PATHOPHYSIOLOGY : concepts of altered health states. [Place of publication not identified]: WOLTERS KLUWER HEALTH. ISBN 978-1496377593. OCLC 1054224262.
- ^ Harrison's principles of internal medicine. Kasper, Dennis L.,, Fauci, Anthony S., 1940-, Hauser, Stephen L.,, Longo, Dan L. (Dan Louis), 1949-, Jameson, J. Larry,, Loscalzo, Joseph (19th ed.). New York. 2015-04-08. ISBN 9780071802154. OCLC 893557976.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ Donal, E; Panis, V (October 2021). "Interaction between mitral valve apparatus and left ventricle. Functional mitral regurgitation: A brief state-of-the-art overview". Advances in Clinical and Experimental Medicine. 30 (10): 991–997. doi:10.17219/acem/143324. PMID 34714608. S2CID 240154628.
- ^ a b c Sinnatamby, Chummy S. (2011). Last's Anatomy (12th ed.). Elsevier Australia. p. 201. ISBN 978-0-7295-3752-0.
- ^ a b Omran, A. S.; Arifi, A. A.; Mohamed, A. A. (2010-07-01). "Echocardiography of the mitral valve". Journal of the Saudi Heart Association. 22 (3): 165–170. doi:10.1016/j.jsha.2010.04.001. ISSN 1016-7315. PMC 3727372. PMID 23960615.
- ^ Lancellotti, P.; Moura, L.; Pierard, L. A.; Agricola, E.; Popescu, B. A.; Tribouilloy, C.; Hagendorff, A.; Monin, J. L.; Badano, L.; Zamorano, J. L.; on behalf of the European Association of Echocardiography (2010-05-01). "European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease)". European Journal of Echocardiography. 11 (4): 307–332. doi:10.1093/ejechocard/jeq031. ISSN 1525-2167. PMID 20435783.
- ^ Nazari S, Carli F, Salvi S, et al. (Apr 2000). "Patterns of systolic stress distribution on mitral valve anterior leaflet chordal apparatus. A structural mechanical theoretical analysis". J Cardiovasc Surg (Torino). 41 (2): 193–202. PMID 10901521.
- ^ Mahmood, Feroze; Shakil, Omair; Mahmood, Bilal; Chaudhry, Maria; Matyal, Robina; Khabbaz, Kamal R. (December 2013). "Mitral Annulus: An Intraoperative Echocardiographic Perspective". Journal of Cardiothoracic and Vascular Anesthesia. 27 (6): 1355–1363. doi:10.1053/j.jvca.2013.02.008. PMID 23962462.
- ^ Perloff, J. K.; Roberts, W. C. (1972). "The Mitral Apparatus: Functional Anatomy of Mitral Regurgitation". Circulation. 46 (2): 227–239. doi:10.1161/01.cir.46.2.227. PMID 5046018.
- ^ Otto, Catherine M.; Bonow, Robert (September 2009). Valvular Heart Disease: A Companion to Braunwald's Heart Disease. Philadelphia: Elsevier Health Sciences. ISBN 9781416058922.
- ^ Pai RG, Varadarajan P, Tanimoto M (2003). "Effect of atrial fibrillation on the dynamics of mitral annular area". The Journal of Heart Valve Disease. 12 (1): 31–7. PMID 12578332. Archived from the original on 2011-07-13. Retrieved 2010-03-04.
- ^ Playford, David; Weyman, Arthur (2001). "Mitral valve prolapse: time for a fresh look". Reviews in Cardiovascular Medicine. 2 (2): 73–81. PMID 12439384. Archived from the original on 2014-09-03. Retrieved 2015-01-01.
- ^ Douedi, Steven; Douedi, Hani (2024), "Mitral Regurgitation", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 31985928, retrieved 2024-11-10
- ^ Séguéla, Pierre-Emmanuel; Houyel, Lucile; Acar, Philippe (2011-08-01). "Congenital malformations of the mitral valve". Archives of Cardiovascular Diseases. 104 (8): 465–479. doi:10.1016/j.acvd.2011.06.004. ISSN 1875-2136. PMID 21944149.
- ^ Salik, Irim; Lee, Lawrence S.; Sharma, Sanjeev; Widrich, Jason (2024), "Mitral Valve Repair", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 31751069, retrieved 2024-11-10
- ^ Kosior, Dariusz A. (2014). "Mitral annulus caseous calcification mimicking cardiac mass in asymptomatic patient – multimodality imaging approach to incidental echocardiographic finding". Polish Journal of Radiology. 79: 88–90. doi:10.12659/PJR.889830. PMC 4005861. PMID 24791181.
- ^ Patrizio Lancellotti, Philippe Pibarot, John Chambers, Giovanni La Canna, Mauro Pepi, Raluca Dulgheru, Mark Dweck, Victoria Delgado, Madalina Garbi, Mani A Vannan, David Montaigne, Luigi Badano, Pal Maurovich-Horvat, Gianluca Pontone, Alec Vahanian, Erwan Donal, Bernard Cosyns, the Scientific Document Committee of the European Association of Cardiovascular Imaging, Multi-modality imaging assessment of native valvular regurgitation: an EACVI and ESC council of valvular heart disease position paper, European Heart Journal - Cardiovascular Imaging, Volume 23, Issue 5, May 2022, Pages e171–e232, https://doi.org/10.1093/ehjci/jeab253
Further reading
edit- Ingels, Neil B.; Karlsson, Matts (21 January 2016). Mitral Valve Mechanics (PDF). Linköping University Electronic Press. ISBN 9789176859520. Archived (PDF) from the original on 19 May 2022.
External links
edit- Anatomy figure: 20:07-03 at Human Anatomy Online, SUNY Downstate Medical Center—"Valves of the heart"
- Cardiac Valve Animations—Perioperative Interactive Education Group