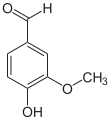
Vanillin is an organic compound with the molecular formula C8H8O3. It is a phenolic aldehyde. Its functional groups include aldehyde, hydroxyl, and ether. It is the primary component of the extract of the vanilla bean. Synthetic vanillin is now used more often than natural vanilla extract as a flavoring in foods, beverages, and pharmaceuticals.
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4-Hydroxy-3-methoxybenzaldehyde | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 472792 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.060 | ||
| EC Number |
| ||
| 3596 | |||
| KEGG | |||
| MeSH | vanillin | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C8H8O3 | |||
| Molar mass | 152.149 g·mol−1 | ||
| Appearance | White solid | ||
| Odor | Vanilla, sweet, balsamic, pleasant | ||
| Density | 1.056 g/cm3[3] | ||
| Melting point | 81 °C (178 °F; 354 K)[3] | ||
| Boiling point | 285 °C (545 °F; 558 K)[3] | ||
| 10 g/L | |||
| log P | 1.208 | ||
| Vapor pressure | >1 Pa | ||
| Acidity (pKa) | 7.781 | ||
| Basicity (pKb) | 6.216 | ||
| Structure | |||
| Monoclinic | |||
| Thermochemistry | |||
Std enthalpy of
combustion (ΔcH⦵298) |
−3.828 MJ/mol | ||
| Hazards | |||
| GHS labelling: | |||

| |||
| Warning | |||
| H302, H317, H319 | |||
| P280, P305+P351+P338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 147 °C (297 °F; 420 K) | ||
| Safety data sheet (SDS) | ICSC 1740 | ||
| Related compounds | |||
Related compounds
|
Anisaldehyde Apocynin Eugenol Phenol Vanillyl alcohol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Vanillin and ethylvanillin are used by the food industry; ethylvanillin is more expensive, but has a stronger note. It differs from vanillin by having an ethoxy group (−O−CH2CH3) instead of a methoxy group (−O−CH3).
Natural vanilla extract is a mixture of several hundred different compounds in addition to vanillin. Artificial vanilla flavoring is often a solution of pure vanillin, usually of synthetic origin. Because of the scarcity and expense of natural vanilla extract, synthetic preparation of its predominant component has long been of interest. The first commercial synthesis of vanillin began with the more readily available natural compound eugenol (4-allyl-2-methoxyphenol). Today, artificial vanillin is made either from guaiacol or lignin.
Lignin-based artificial vanilla flavoring is alleged to have a richer flavor profile than that from guaiacol-based artificial vanilla; the difference is due to the presence of acetovanillone, a minor component in the lignin-derived product that is not found in vanillin synthesized from guaiacol.[4]
History
editAlthough it is generally accepted that vanilla was domesticated in Mesoamerica and subsequently spread to the Old World in the 16th century, in 2019, researchers published a paper stating that vanillin residue had been discovered inside jars within a tomb in Israel dating to the 2nd millennium BCE, suggesting the possible cultivation of an unidentified, Old World-endemic Vanilla species in Canaan since the Middle Bronze Age.[5][6] Traces of vanillin were also found in wine jars in Jerusalem, which were used by the Judahite elite before the city was destroyed in 586 BCE.[6]
Vanilla beans, called tlilxochitl, were discovered and cultivated as a flavoring for beverages by native Mesoamerican peoples, most famously the Totonacs of modern-day Veracruz, Mexico. Since at least the early 15th century, the Aztecs used vanilla as a flavoring for chocolate in drinks called xocohotl.[7]
Vanillin was first isolated as a relatively pure substance in 1858 by Théodore Nicolas Gobley, who obtained it by evaporating a vanilla extract to dryness and recrystallizing the resulting solids from hot water.[8] In 1874, the German scientists Ferdinand Tiemann and Wilhelm Haarmann deduced its chemical structure, at the same time finding a synthesis for vanillin from coniferin, a glucoside of isoeugenol found in pine bark.[9] Tiemann and Haarmann founded a company Haarmann and Reimer (now part of Symrise) and started the first industrial production of vanillin using their process (now known as the Reimer–Tiemann reaction) in Holzminden, Germany. In 1876, Karl Reimer synthesized vanillin (2) from guaiacol (1).[10]
By the late 19th century, semisynthetic vanillin derived from the eugenol found in clove oil was commercially available.[11]
Synthetic vanillin became significantly more available in the 1930s, when production from clove oil was supplanted by production from the lignin-containing waste produced by the sulfite pulping process for preparing wood pulp for the paper industry. By 1981, a single pulp and paper mill in Thorold, Ontario, supplied 60% of the world market for synthetic vanillin.[12] However, subsequent developments in the wood pulp industry have made its lignin wastes less attractive as a raw material for vanillin synthesis. Today, approximately 15% of the world's production of vanillin is still made from lignin wastes,[13] while approximately 85% is synthesized in a two-step process from the petrochemical precursors guaiacol and glyoxylic acid.[14][15]
Beginning in 2000, Rhodia began marketing biosynthetic vanillin prepared by the action of microorganisms on ferulic acid extracted from rice bran. This product, sold at USD$700/kg under the trademarked name Rhovanil Natural, is not cost-competitive with petrochemical vanillin, which sells for around US$15/kg.[16] However, unlike vanillin synthesized from lignin or guaiacol, it can be labeled as a natural flavoring.
Occurrence
editVanillin is most prominent as the principal flavor and aroma compound in vanilla. Cured vanilla pods contain about 2% by dry weight vanillin. Relatively pure vanillin may be visible as a white dust or "frost" on the exteriors of cured pods of high quality.
It is also found in Leptotes bicolor, a species of orchid native to Paraguay and southern Brazil,[17] and the Southern Chinese red pine.
At lower concentrations, vanillin contributes to the flavor and aroma profiles of foodstuffs as diverse as olive oil,[18] butter,[19] raspberry,[20] and lychee[21] fruits.
Aging in oak barrels imparts vanillin to some wines, vinegar,[22] and spirits.[23]
In other foods, heat treatment generates vanillin from other compounds. In this way, vanillin contributes to the flavor and aroma of coffee,[24][25] maple syrup,[26] and whole-grain products, including corn tortillas[27] and oatmeal.[28]
Chemistry
editNatural production
editNatural vanillin is extracted from the seed pods of Vanilla planifolia, a vining orchid native to Mexico, but now grown in tropical areas around the globe. Madagascar is presently the largest producer of natural vanillin.
As harvested, the green seed pods contain vanillin in the form of its β-D-glucoside; the green pods do not have the flavor or odor of vanilla.[29]
After being harvested, their flavor is developed by a months-long curing process, the details of which vary among vanilla-producing regions, but in broad terms it proceeds as follows:
First, the seed pods are blanched in hot water, to arrest the processes of the living plant tissues. Then, for 1–2 weeks, the pods are alternately sunned and sweated: during the day they are laid out in the sun, and each night wrapped in cloth and packed in airtight boxes to sweat. During this process, the pods become dark brown, and enzymes in the pod release vanillin as the free molecule. Finally, the pods are dried and further aged for several months, during which time their flavors further develop. Several methods have been described for curing vanilla in days rather than months, although they have not been widely developed in the natural vanilla industry,[30] with its focus on producing a premium product by established methods, rather than on innovations that might alter the product's flavor profile.
Biosynthesis
editAlthough the exact route of vanillin biosynthesis in V. planifolia is currently unknown, several pathways are proposed for its biosynthesis. Vanillin biosynthesis is generally agreed to be part of the phenylpropanoid pathway starting with L-phenylalanine,[31] which is deaminated by phenylalanine ammonia lyase (PAL) to form t-cinnamic acid. The para position of the ring is then hydroxylated by the cytochrome P450 enzyme cinnamate 4-hydroxylase (C4H/P450) to create p-coumaric acid.[32] Then, in the proposed ferulate pathway, 4-hydroxycinnamoyl-CoA ligase (4CL) attaches p-coumaric acid to coenzyme A (CoA) to create p-coumaroyl CoA. Hydroxycinnamoyl transferase (HCT) then converts p-coumaroyl CoA to 4-coumaroyl shikimate/quinate. This subsequently undergoes oxidation by the P450 enzyme coumaroyl ester 3’-hydroxylase (C3’H/P450) to give caffeoyl shikimate/quinate. HCT then exchanges the shikimate/quinate for CoA to create caffeoyl CoA, and 4CL removes CoA to afford caffeic acid. Caffeic acid then undergoes methylation by caffeic acid O-methyltransferase (COMT) to give ferulic acid. Finally, vanillin synthase hydratase/lyase (vp/VAN) catalyzes hydration of the double bond in ferulic acid followed by a retro-aldol elimination to afford vanillin.[32] Vanillin can also be produced from vanilla glycoside with the additional final step of deglycosylation.[29] In the past p-hydroxybenzaldehyde was speculated to be a precursor for vanillin biosynthesis. However, a 2014 study using radiolabelled precursor indicated that p-hydroxybenzaldehyde is not used to synthesise vanillin or vanillin glucoside in the vanilla orchids.[32]
Chemical synthesis
editThe demand for vanilla flavoring has long exceeded the supply of vanilla beans. As of 2001[update], the annual demand for vanillin was 12,000 tons, but only 1,800 tons of natural vanillin were produced.[33] The remainder was produced by chemical synthesis. Vanillin was first synthesized from eugenol (found in oil of clove) in 1874–75, less than 20 years after it was first identified and isolated. Vanillin was commercially produced from eugenol until the 1920s.[34] Later it was synthesized from lignin-containing "brown liquor", a byproduct of the sulfite process for making wood pulp.[12] Counterintuitively, though it uses waste materials, the lignin process is no longer popular because of environmental concerns, and today most vanillin is produced from guaiacol.[12] Several routes exist for synthesizing vanillin from guaiacol.[35]
At present, the most significant of these is the two-step process practiced by Rhodia since the 1970s, in which guaiacol (1) reacts with glyoxylic acid by electrophilic aromatic substitution.[36] The resulting vanillylmandelic acid (2) is then converted by 4-Hydroxy-3-methoxyphenylglyoxylic acid (3) to vanillin (4) by oxidative decarboxylation.[14]
Wood-based vanillin
edit15% of the world's production of vanillin is produced from lignosulfonates, a byproduct from the manufacture of cellulose via the sulfite process.[12][13] The sole producer of wood-based vanillin is the company Borregaard located in Sarpsborg, Norway.
Wood-based vanillin is produced by copper-catalyzed oxidation of the lignin structures in lignosulfonates under alkaline conditions[37] and is claimed by the manufacturing company to be preferred by their customers due to, among other reasons, its much lower carbon footprint than petrochemically synthesized vanillin.
Fermentation
editThe company Evolva has developed a genetically modified microorganism which can produce vanillin. Because the microbe is a processing aid, the resulting vanillin would not fall under U.S. GMO labeling requirements, and because the production is nonpetrochemical, food using the ingredient can claim to contain "no artificial ingredients".[38]
Using ferulic acid as an input and a specific non GMO species of Amycolatopsis bacteria, natural vanillin can be produced.
Biochemistry
editSeveral studies have suggested that vanillin can affect the performance of antibiotics in laboratory conditions.[39][40]
Uses
editThe largest use of vanillin is as a flavoring, usually in sweet foods. The ice cream and chocolate industries together comprise 75% of the market for vanillin as a flavoring, with smaller amounts being used in confections and baked goods.[41]
Vanillin is also used in the fragrance industry, in perfumes, and to mask unpleasant odors or tastes in medicines, livestock fodder, and cleaning products.[14] It is also used in the flavor industry, as a very important key note for many different flavors, especially creamy profiles such as cream soda.
Additionally, vanillin can be used as a general-purpose stain for visualizing spots on thin-layer chromatography plates. This stain yields a range of colors for these different components.
Vanillin–HCl staining can be used to visualize the localisation of tannins in cells.
Vanillin is becoming a popular choice for the development of bio-based plastics.[42]
Manufacturing
editVanillin has been used as a chemical intermediate in the production of pharmaceuticals, cosmetics, and other fine chemicals.[43] In 1970, more than half the world's vanillin production was used in the synthesis of other chemicals.[12] As of 2016, vanillin uses have expanded to include perfumes, flavoring and aromatic masking in medicines, various consumer and cleaning products, and livestock foods.[44]
Adverse effects
editVanillin can trigger migraine headaches in a small fraction of the people who experience migraines.[45]
Some people have allergic reactions to vanilla.[46] They may be allergic to synthetically produced vanilla but not to natural vanilla, or the other way around, or to both.[47]
Vanilla orchid plants can trigger contact dermatitis, especially among people working in the vanilla trade if they come into contact with the plant's sap.[47] An allergic contact dermatitis called vanillism produces swelling and redness, and sometimes other symptoms.[47] The sap of most species of vanilla orchid which exudes from cut stems or where beans are harvested can cause moderate to severe dermatitis if it comes in contact with bare skin. The sap of vanilla orchids contains calcium oxalate crystals, which are thought to be the main causative agent of contact dermatitis in vanilla plantation workers.[48][49]
A pseudophytodermatitis called vanilla lichen can be caused by flour mites (Tyroglyphus farinae).[47]
Ecology
editScolytus multistriatus, one of the vectors of the Dutch elm disease, uses vanillin as a signal to find a host tree during oviposition.[50]
See also
editReferences
edit- Blank, Imre; Alina Sen; Werner Grosch (1992). "Potent odorants of the roasted powder and brew of Arabica coffee". Zeitschrift für Lebensmittel-Untersuchung und -Forschung A. 195 (3): 239–245. doi:10.1007/BF01202802. S2CID 67845142.
- Bjørsvik, Hans-René; Minisci, Franscesco (1999). "Fine Chemicals from Lignosulfonates. 1. Synthesis of Vanillin by Oxidation of Lignosulfonates". Org. Process Res. Dev. 3 (5): 330–340. doi:10.1021/op9900028.
- Brenes, Manuel; Aranzazu García; Pedro García; José J. Rios; Antonio Garrido (1999). "Phenolic Compounds in Spanish Olive Oils". Journal of Agricultural and Food Chemistry. 47 (9): 3535–3540. doi:10.1021/jf990009o. PMID 10552681.
- Buttery, Ron G.; Louisa C. Ling (1995). "Volatile Flavor Components of Corn Tortillas and Related Products". Journal of Agricultural and Food Chemistry. 43 (7): 1878–1882. doi:10.1021/jf00055a023.
- Dignum, Mark J. W.; Josef Kerlera; Rob Verpoorte (2001). "Vanilla Production: Technological, Chemical, and Biosynthetic Aspects". Food Reviews International. 17 (2): 119–120. doi:10.1081/FRI-100000269. S2CID 84296900. Retrieved 2006-09-09.
- Esposito, Lawrence J.; K. Formanek; G. Kientz; F. Mauger; V. Maureaux; G. Robert; F. Truchet (1997). "Vanillin". Kirk-Othmer Encyclopedia of Chemical Technology, 4th edition. Vol. 24. New York: John Wiley & Sons. pp. 812–825. ISBN 978-0-471-52693-3.
- Fache, Maxence; Boutevin, Bernard; Caillol, Sylvain (2015). "Vanillin Production from Lignin and Its Use as a Renewable Chemical". ACS Sustain. Chem. Eng. 4 (1): 35–46. doi:10.1021/acssuschemeng.5b01344.
- Fund for Research into Industrial Development, Growth and Equity (FRIDGE) (2004). Study into the Establishment of an Aroma and Fragrance Fine Chemicals Value Chain in South Africa, Part Three: Aroma Chemicals Derived from Petrochemical Feedstocks. National Economic Development and Labor Council. Archived from the original on 2007-09-30. Retrieved 2017-07-08.
- Gobley, M. (1858). "Recherches sur le principe odorant de la vanille". Journal de Pharmacie et de Chimie. 34: 401–405.
- Guth, Helmut; Werner Grosch (1995). "Odorants of extrusion products of oat meal: Changes during storage". Zeitschrift für Lebensmittel-Untersuchung und -Forschung A. 196 (1): 22–28. doi:10.1007/BF01192979. S2CID 82716730.
- Hocking, Martin B. (September 1997). "Vanillin: Synthetic Flavoring from Spent Sulfite Liquor" (PDF). Journal of Chemical Education. 74 (9): 1055–1059. Bibcode:1997JChEd..74.1055H. doi:10.1021/ed074p1055. Retrieved 2006-09-09.
- Kermasha, S.; M. Goetghebeur; J. Dumont (1995). "Determination of Phenolic Compound Profiles in Maple Products by High-Performance Liquid Chromatography". Journal of Agricultural and Food Chemistry. 43 (3): 708–716. doi:10.1021/jf00051a028.
- Lampman, Gary M.; Jennifer Andrews; Wayne Bratz; Otto Hanssen; Kenneth Kelley; Dana Perry; Anthony Ridgeway (1977). "Preparation of vanillin from eugenol and sawdust". Journal of Chemical Education. 54 (12): 776–778. Bibcode:1977JChEd..54..776L. doi:10.1021/ed054p776.
- Ong, Peter K. C.; Terry E. Acree (1998). "Gas Chromatography/Olfactory Analysis of Lychee (Litchi chinesis Sonn.)". Journal of Agricultural and Food Chemistry. 46 (6): 2282–2286. doi:10.1021/jf9801318.
- Reimer, Karl Ludwig (1876). "Ueber eine neue Bildungsweise aromatischer Aldehyde". Berichte der Deutschen Chemischen Gesellschaft. 9 (1): 423–424. doi:10.1002/cber.187600901134.
- Roberts, Deborah D.; Terry E. Acree (1996). "Effects of Heating and Cream Addition on Fresh Raspberry Aroma Using a Retronasal Aroma Simulator and Gas Chromatography Olfactometry". Journal of Agricultural and Food Chemistry. 44 (12): 3919–3925. doi:10.1021/jf950701t.
- Rouhi, A. Maureen (2003). "Fine Chemicals Firms Enable Flavor And Fragrance Industry". Chemical and Engineering News. 81 (28): 54.
- Tiemann, Ferd.; Wilh. Haarmann (1874). "Ueber das Coniferin und seine Umwandlung in das aromatische Princip der Vanille". Berichte der Deutschen Chemischen Gesellschaft. 7 (1): 608–623. doi:10.1002/cber.187400701193.
- Van Ness, J. H. (1983). "Vanillin". Kirk-Othmer Encyclopedia of Chemical Technology, 3rd edition. Vol. 23. New York: John Wiley & Sons. pp. 704–717. ISBN 978-0-471-02076-9.
- Viriot, Carole; Augustin Scalbert; Catherine Lapierre; Michel Moutounet (1993). "Ellagitannins and lignins in aging of spirits in oak barrels". Journal of Agricultural and Food Chemistry. 41 (11): 1872–1879. doi:10.1021/jf00035a013.
- Vreuls, René J. J.; van der Heijden, Arnold; Brinkman, Udo A. Th.; Adahchour, Mohamed (1999). "Trace-level determination of polar flavour compounds in butter by solid-phase extraction and gas chromatography–mass spectrometry". Journal of Chromatography A. 844 (1–2): 295–305. doi:10.1016/S0021-9673(99)00351-9. PMID 10399332.
- Walton, Nicholas J.; Melinda J. Mayer; Arjan Narbad (July 2003). "Vanillin". Phytochemistry. 63 (5): 505–515. Bibcode:2003PChem..63..505W. doi:10.1016/S0031-9422(03)00149-3. PMID 12809710.
Notes
edit- ^ a b Field, Simon Quellen. "Vanillin". sci-toys.com.
- ^ CID 1183 from PubChem.
- ^ a b c Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 3.310. ISBN 978-1-4987-5429-3.
- ^ According to Esposito 1997, blind taste-testing panels cannot distinguish between the flavors of synthetic vanillin from lignin and those from guaicol, but can distinguish the odors of these two types of synthetic vanilla extracts. Guaiacol vanillin, adulterated with acetovanillone, has an odor indistinguishable from lignin vanillin.
- ^ Linares, V.; Adams, M. J.; Cradic, M. S.; Finkelstein, I.; Lipschits, O.; Martin, M. A. S.; Neumann, R.; Stockhammer, P. W.; Gadot, Y. (June 2019). "First evidence for vanillin in the old world: Its use as mortuary offering in Middle Bronze Canaan". Journal of Archaeological Science: Reports. 25: 77–84. Bibcode:2019JArSR..25...77L. doi:10.1016/j.jasrep.2019.03.034. S2CID 181608839.
- ^ a b Amir, A.; Finkelstein, I.; Shalev, Y.; Uziel, J.; Chalaf, O.; Freud, L.; Neumann, R.; Gadot, Y. (2022). "Amir A, Finkelstein I, Shalev Y, Uziel J, Chalaf O, Freud L, et al. (2022) Residue analysis evidence for wine enriched with vanilla consumed in Jerusalem on the eve of the Babylonian destruction in 586 BCE. PLoS ONE 17(3)". PLOS ONE. 17 (3): e0266085. doi:10.1371/journal.pone.0266085. PMC 8963535. PMID 35349581.
- ^ MexicanVanilla.com. "Mexican Vanilla - A History". MexicanVanilla.com. Retrieved 2022-06-14.
- ^ Gobley 1858.
- ^ Tiemann 1874.
- ^ Reimer 1876.
- ^ According to Hocking 1997, synthetic vanillin was sold commercially in 1874, the same year Tiemann and Haarmann's original synthesis was published. Haarmann and Reimer, one of the corporate ancestors of the modern flavor and aroma manufacturer Symrise, was in fact established in 1874. However, Esposito 1997 claims that synthetic vanillin first became available in 1894 when Rhône-Poulenc (since 1998, Rhodia) entered the vanillin business. If the former claim is correct, the authors of the latter article, being employees of Rhône-Poulenc, may have been unaware of any previous vanillin manufacture.
- ^ a b c d e Hocking 1997.
- ^ a b Fache et al 2015
- ^ a b c Esposito 1997.
- ^ Kamlet, Jonas & Mathieson, Olin (1953). Manufacture of vanillin and its homologues U.S. Patent 2,640,083 (PDF). U.S. Patent Office.
- ^ Rouhi 2003.
- ^ "Leptotes bicolor". Flora Library. Retrieved 2011-08-21.
- ^ Brenes 1999.
- ^ Adahchour 1999.
- ^ Roberts 1996.
- ^ Ong 1998.
- ^ Carrero Gálvez, Miguel (1994). "Analysis of polyphenolic compounds of different vinegar samples". Zeitschrift für Lebensmittel-Untersuchung und -Forschung. 199: 29–31. doi:10.1007/BF01192948. S2CID 91784893..
- ^ Viriot 1993.
- ^ Semmelroch, P.; Laskawy, G.; Blank, I.; Grosch, W. (1995). "Determination of potent odourants in roasted coffee by stable isotope dilution assays". Flavour and Fragrance Journal. 10: 1–7. doi:10.1002/ffj.2730100102.
- ^ Blank 1992.
- ^ Kermasha 1995.
- ^ Buttery 1995.
- ^ Guth 1993.
- ^ a b Walton 2003.
- ^ Dignum 2001 reviews several such proposed innovations in vanilla processing, including processes in which the seed pods are chopped, frozen, warmed by a heat source other than the sun, or crushed and treated by various enzymes. Whether or not these procedures produce a product whose taste is comparable to traditionally prepared natural vanilla, many of them are incompatible with the customs of the natural vanilla market, in which the vanilla beans are sold whole, and graded by, among other factors, their length.
- ^ Dixon, R. A. (2014). "Vanillin Biosynthesis – Not as simple as it seems?" (PDF). Handbook of Vanilla Science and Technology: 292.
- ^ a b c Gallage, N. J.; Hansen, E. H.; Kannangara, R.; Olsen, E. C.; Motawia, M. S.; Jørgensen, K.; Holme, I.; Hebelstrup, K.; Grisoni, M.; Møller, L. B. (2014). "Vanillin formation from ferulic acid in Vanilla planifolia is catalysed by a single enzyme". Nature Communications. 5: 4037. Bibcode:2014NatCo...5.4037G. doi:10.1038/ncomms5037. PMC 4083428. PMID 24941968.
- ^ Dignum 2001.
- ^ Hocking 1997. This chemical process can be conveniently carried out on the laboratory scale using the procedure described by Lampman 1977.
- ^ Van Ness 1983.
- ^ Fatiadi, Alexander & Schaffer, Robert (1974). "An Improved Procedure for Synthesis of DL-4-Hydroxy-3-methoxymandelic Acid (DL-"Vanillyl"-mandelic Acid, VMA)". Journal of Research of the National Bureau of Standards Section A. 78A (3): 411–412. doi:10.6028/jres.078A.024. PMC 6742820. PMID 32189791.
- ^ Bjørsvik and Minisci 1999
- ^ Bomgardner, Melody M. (2016-09-14). "The Problem with Vanilla". Scientific American. Retrieved 2020-10-19.
- ^ Brochado, Ana (4 July 2018). "Species-specific activity of antibacterial drug combinations". Nature. 559 (7713): 259–263. Bibcode:2018Natur.559..259B. doi:10.1038/s41586-018-0278-9. PMC 6219701. PMID 29973719.
- ^ Bezzera, Camila (1 December 2017). "Vanillin selectively modulates the action of antibiotics against resistant bacteria". Microbial Pathogenesis. 113: 265–268. doi:10.1016/j.micpath.2017.10.052. PMID 29107747.
- ^ FRIDGE 2004, p. 33.
- ^ Fache, Maxence; Boutevin, Bernard; Caillol, Sylvain (2015). "Vanillin, a key-intermediate of biobased polymers". European Polymer Journal. 68: 488–502. Bibcode:2015EurPJ..68..488F. doi:10.1016/j.eurpolymj.2015.03.050.
- ^ Sinha, A. K.; Sharma, U. K.; Sharma, N. (2008). "A comprehensive review on vanilla flavor: Extraction, isolation and quantification of vanillin and others constituents". International Journal of Food Sciences and Nutrition. 59 (4): 299–326. doi:10.1080/09687630701539350. PMID 17886091. S2CID 37559260.
- ^ "Global Vanillin Market Research Report – Industry Analysis, Size, Share, Growth, Trends and Forecast 2015–2022". PRNewsire. 14 September 2016. Retrieved 18 February 2017.
- ^ Saint Denis, M.; Coughtrie, M. W.; Guilland, J. C.; Verges, B.; Lemesle, M.; Giroud, M. (Dec 1996). "Migraine induced by vanillin". Presse Méd. 25 (40): 2043. PMID 9082382.
- ^ Van Assendelft, A. H. (1987). "Adverse drug reactions checklist". British Medical Journal (Clinical Research Ed.). 294 (6571): 576–577. doi:10.1136/bmj.294.6571.576-d. PMC 1245616. PMID 3103791.
- ^ a b c d Rietschel, Robert L.; Fowler, Joseph F.; Fisher, Alexander A. (2008). Fisher's Contact Dermatitis. PMPH-USA. p. 444. ISBN 978-1-55009-378-0.
- ^ "Vanilla planifolia Andrews - Plants of the World Online - Kew Science". powo.science.kew.org. Archived from the original on 22 November 2017.
- ^ "Vanillin". Plants for a Future. Archived from the original on 1 December 2017.
- ^ Meyer, H.J.; Norris, D.M. (17 July 1967). "Vanillin and Syringaldehyde as Attractants for Scolytus multistriatus (Coleoptera: Scolytidae)". Annals of the Entomological Society of America. 60 (4): 858–859. doi:10.1093/aesa/60.4.858.