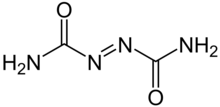
Azodicarbonamide, ADCA, ACA,[1] ADA, or azo(bis)formamide, is a chemical compound with the molecular formula C2H4O2N4.[2] It is a yellow to orange-red, odorless, crystalline powder. It is sometimes called a 'yoga mat' chemical because of its widespread use in foamed plastics.[3][4] It was first described by John Bryden in 1959.[5]
| |

| |
| Names | |
|---|---|
| IUPAC name
Carbamoyliminourea
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.229 |
| EC Number |
|
| E number | E927a (glazing agents, ...) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H4N4O2 | |
| Molar mass | 116.080 g·mol−1 |
| Appearance | Yellow to orange/red crystalline powder |
| Melting point | 225 °C (437 °F; 498 K) (decomposes) |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H242, H331, H334 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
editIt is prepared in two steps via treatment of urea with hydrazine to form biurea, as described in this idealized equation:
- 2 O=C(NH2)2 + H2N−NH2 → H2N−C(=O)−NH−NH−C(=O)−NH2 + 2 NH3
Oxidation with chlorine or chromic acid yields azodicarbonamide:
- H2N−C(=O)−NH−NH−C(=O)−NH2 + Cl2 → H2N−C(=O)−N=N−C(=O)−NH2 + 2 HCl
Applications
editBlowing agent
editThe principal use of azodicarbonamide is in the production of foamed plastics as a blowing agent. The thermal decomposition of azodicarbonamide produces nitrogen, carbon monoxide, carbon dioxide, and ammonia gases, which are trapped in the polymer as bubbles to form a foamed article.[6]
Azodicarbonamide is used in plastics, synthetic leather, and other industries and can be pure or modified. Modification affects the reaction temperatures. Pure azodicarbonamide generally reacts around 200 °C. In the plastic, leather, and other industries, modified azodicarbonamide (average decomposition temperature 170 °C) contains additives that accelerate the reaction or react at lower temperatures.[6]
An example of the use of azodicarbonamide as a blowing agent is found in the manufacture of vinyl (PVC) and EVA-PE foams, where it forms bubbles upon breaking down into gas at high temperature. Vinyl foam is springy and does not slip on smooth surfaces. It is useful for carpet underlay and floor mats. Commercial yoga mats made of vinyl foam have been available since the 1980s; the first mats were cut from carpet underlay.[7]
Food additive
editAs a food additive, azodicarbonamide is used as a flour bleaching agent and a dough conditioner.[8] It reacts with moist flour as an oxidizing agent.[9] The main reaction product is biurea, which is stable during baking.[9] Secondary reaction products include semicarbazide and ethyl carbamate.[8] It is known by the E number E927. Many restaurants in the US fast food industry removed the additive in response to negative publicity.[10]
Safety and regulation
editOccupational (inhalation)
editIn a 1999 report, the World Health Organization has linked exposure to azodicarbonamide at workplaces where it is manufactured or handled in raw form to "respiratory issues, allergies and asthma". The available data are restricted to these occupational environments. Exposure of the general public to azodicarbonamide could not be evaluated because of the lack of available data.[11] The WHO concluded, "The level of risk is uncertain; hence, exposure levels should be reduced as much as possible".
In the UK, the Health and Safety Executive has identified azodicarbonamide as a respiratory sensitizer (a possible cause of asthma) in workplace settings and determined that containers of it should be labeled with "May cause sensitisation by inhalation."[12] Azodicarbonamide was added to the REACH Regulation candidate Substances of Very High Concern list in 2012, for its respiratory sensitizing properties.[13]
Food (ingestion)
editIn some jurisdictions, the use of azodicarbonamide as a flour bleaching agent has been phased out. For example, it is no longer authorized for use in Australia and the European Union as a food additive.[14][15] Azodicarbonamide as a blowing agent in plastics has been banned in the European Union since August 2005 for the manufacture of plastic articles that are intended to come into direct contact with food.[16] In the United States, azodicarbonamide has a generally recognized as safe (GRAS) status and is allowed to be added to flour at levels up to 45 ppm.[17][14] However, use in products intended for human consumption is in decline under pressure of the public opinion.[10] In 2014, amid public discomfort with the dual uses of azodicarbonamide, the sandwich franchise Subway and hamburger franchise Wendy's announced that they would no longer use it as a dough conditioner.[18] As of February 2014, the Center for Science in the Public Interest stated azodicarbonamide "has been poorly tested" and advocates for reducing the amount of azodicarbonamide that is allowed to be used in food.[18]
Banning ADA in food is motivated by studies of semicarbazide, a side product of ADA use in packaging, showing "weak carcinogenic activity in laboratory animals" but inconclusive as to carcinogenic risk to humans.[16] The EU banned ADA in food containers despite an EFSA report considering such exposure "not a concern" due to low levels produced. The FDA considers ADA to be safe in permissible concentrations.[8]
As of February, 2021, in contrast to direct competitors like Wendy's that have phased out the ingredient, A&W continues to use azodicarbonamide in an unspecified quantity ("under 2 %") in their standard hamburger buns.[19]
References
edit- ^ Farah, Troy (28 May 2019). "Banned bread: why does the US allow additives that Europe says are unsafe?". The Guardian. ISSN 0261-3077. Retrieved 7 June 2024.
- ^ "Azodicarbonamide (CICADS)". Inchem. International Programme on Chemical Safety. Archived from the original on 24 August 2010. Retrieved 14 August 2010. Also published by World Health Organization, Geneva, 1999.
- ^ Arts, Josje; Kimber, Ian (October 2017). "Azodicarbonamide (ADCA): A reconsideration of classification as a respiratory sensitiser". Regulatory Toxicology and Pharmacology. 89: 268–278. doi:10.1016/j.yrtph.2017.07.018. PMID 28734852.
- ^ "Almost 500 Foods Contain the 'Yoga Mat' Compound. Should We Care?". NPR.
- ^ Bryden, J. H. (10 January 1961). "The crystal structure of azodicarbonamide". Acta Crystallographica. 14 (1): 61–63. Bibcode:1961AcCry..14...61B. doi:10.1107/S0365110X61000139.
- ^ a b Heinz Weber; Isidoor De Grave; Eckhard Röhrl; Volker Altstädt (2016). "Foamed Plastics". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. pp. 1–54. doi:10.1002/14356007.a11_435.pub2. ISBN 978-3-527-30673-2.
- ^ Friend, John (2009). History of Yoga Mat - Looking back with Friends.
- ^ a b c FDA Frequently Asked Questions on Azodicarbonamide (ADA) Page Last Updated: 20 June 2014
- ^ a b WHO FAO 1965. Toxicological Evaluation of Some Antimicrobials, Antioxidants, Emulsifiers, Stabilizers, Flour-Treatment Agents, Acids and Bases: Azodicarbonamide FAO Nutrition Meetings Report Series No. 40A,B,C. WHO/Food Add./67.29
- ^ a b "The Yoga-Mat Chemical's Quiet Fast-Food Exit". Bloomberg. 8 August 2016. Retrieved 8 August 2016.
- ^ "Concise International Chemical Assessment Document 16: Azodicarbonamide" (PDF). World Health Organization. Retrieved 5 February 2014.
- ^ "Substances causing/worsening asthma". UK Occupational Health and Safety. WorkSafe Victoria. Archived from the original on 31 May 2013. Retrieved 14 August 2010.
- ^ "Candidate List of substances of very high concern for Authorisation". ECHA. Retrieved 30 October 2018.
Diazene-1,2-dicarboxamide (C,C'-azodi(formamide)) (ADCA)... respiratory sensitising properties (Article 57(f) - human health)
- ^ a b Smith, Jim; Hong-Shum, Lily (2011). Food additives data book (2nd ed.). Chichester, West Sussex: Wiley-Blackwell. p. 548. ISBN 978-1-4051-9543-0.
- ^ European Commission. "European Union: Authorisation of Additives". Archived from the original on 31 December 2014. Retrieved 31 December 2014.
- ^ a b "COMMISSION DIRECTIVE 2004/1/EC of 6 January 2004 amending Directive 2002/72/EC as regards the suspension of the use of azodicarbonamide as blowing agent". Official Journal of the European Union. 13 January 2004. Retrieved 10 March 2011.
- ^ "21CFR172.806". Code of Federal Regulations. 1 April 2012.
- ^ a b Landau, Elizabeth (17 February 2014). "Subway to remove 'dough conditioner' chemical from bread". CNN. Retrieved 3 November 2015.
- ^ All American Foodawrestaurants.com Archived 22 April 2022 at the Wayback Machine
