The Algar–Flynn–Oyamada reaction is a chemical reaction whereby a chalcone undergoes an oxidative cyclization to form a flavonol.[1][2]
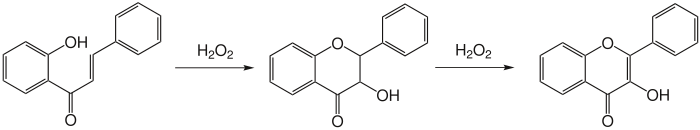
Reaction mechanism
editThere are several possible mechanisms to explain this reaction; however, these reaction mechanisms have not been elucidated. What is known is that a two-stage mechanism exists. First, dihydroflavonol is formed, which then subsequently oxidizes to form a flavonol.
Proposed mechanisms involving epoxidation of the alkene have been disproven.[3]
The probable mechanisms are thus two possibilities:
- The phenoxide attacks the enone at the beta position, and the alkene directly attacks hydrogen peroxide from the alpha position, forming the dihydroflavonol.
- The phenoxide attacks the enone at the beta position, closing the six-membered ring and forming an enolate intermediate. The enolate then attacks hydrogen peroxide, forming the dihydroflavonol.
See also
editReferences
edit- ^ Algar, J.; Flynn, J. P. (1934). Proceedings of the Royal Irish Academy. 42B: 1.
{{cite journal}}: CS1 maint: untitled periodical (link) - ^ Oyamada, B. (1935). "A New General Method for the Synthesis of the Derivatives of Flavonol". Bulletin of the Chemical Society of Japan. 10 (5): 182–186. doi:10.1246/bcsj.10.182.
- ^ Gormley, T.R.; O'Sullivan, W.I. (1973). "Flavanoid epoxides—XIII". Tetrahedron. 29 (2): 369–373. doi:10.1016/S0040-4020(01)93304-6. hdl:10197/6996. ISSN 0040-4020.