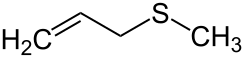
Allyl methyl sulfide is an organosulfur compound with the chemical formula CH2=CHCH2SCH3. The molecule features two functional groups, an allyl (CH2=CHCH2) and a sulfide. It is a colourless liquid with a strong odor characteristic of alkyl sulfides. It is a metabolite of garlic, and "garlic breath" is attributed to its presence.[1]
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-(Methylsulfanyl)prop-1-ene | |
| Other names
Methyl propenyl sulfide
3-Methylthio-1-propene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.371 |
| EC Number |
|
| MeSH | allyl+methyl+sulfide |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1993 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H8S | |
| Molar mass | 88.17 g·mol−1 |
| Odor | Garlic |
| Density | 0.803 g cm−3 |
| Boiling point | 92 °C; 197 °F; 365 K |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H225 | |
| P210 | |
| Flash point | 18.0 °C (64.4 °F; 291.1 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
It is prepared by the reaction of allyl chloride with sodium hydroxide and methanethiol.
- CH2=CHCH2Cl + NaOH (aq) + CH3SH → CH2=CHCH2SCH3 + NaCl + H2O
References
edit- ^ Eric Block (2010-01-04). Garlic and Other Alliums: The Lore and the Science. Royal Society of Chemistry. ISBN 978-0-85404-190-9.